Abstract
Purpose
In order to facilitate targeted outreach, we sought to identify patient populations with a lower likelihood of returning for breast cancer screening after COVID-19-related imaging center closures.
Methods
Weekly total screening mammograms performed throughout 2019 (baseline year) and 2020 (COVID-19-impacted year) were compared. Demographic and clinical characteristics, including age, race, ethnicity, breast density, breast cancer history, insurance status, imaging facility type used, and need for interpreter, were compared between patients imaged from March 16 to October 31 in 2019 (baseline cohort) and 2020 (COVID-19-impacted cohort). Census data and an online map service were used to impute socioeconomic variables and calculate travel times for each patient. Logistic regression was used to identify patient characteristics associated with a lower likelihood of returning for screening after COVID-19-related closures.
Results
The year-over-year cumulative difference in screening mammogram volumes peaked in week 21, with 2962 fewer exams in the COVID-19-impacted year. By week 47, this deficit had reduced by 49.4% to 1498. A lower likelihood of returning for screening after COVID-19-related closures was independently associated with younger age (odds ratio (OR) 0.78, p < 0.001), residence in a higher poverty area (OR 0.991, p = 0.014), lack of health insurance (OR 0.65, p = 0.007), need for an interpreter (OR 0.68, p = 0.029), longer travel time (OR 0.998, p < 0.001), and utilization of mobile mammography services (OR 0.27, p < 0.001).
Conclusion
Several patient factors are associated with a lower likelihood of returning for screening mammography after COVID-19-related closures. Knowledge of these factors can guide targeted outreach to vulnerable patients to facilitate breast cancer screening.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-021-06252-1.
Keywords: Screening mammography, COVID-19 pandemic, Breast cancer, Patient travel time
Introduction
The beginning of the COVID-19 pandemic in March 2020 prompted government and health organizations to implement policies aimed at decreasing the spread of the virus among patients and providers. These strategies and patient perceptions of the safety of in-person medical care led to a significant decline in medical encounters [1, 2]. Non-emergent outpatient radiology services were among the most commonly postponed encounters during the early stages of the COVID-19 pandemic [1] as the effort to limit the spread of the virus necessitated triaging patients by weighing the risks of delaying care against the risks of potential exposure to COVID-19.
Mammographic breast cancer screening, an outpatient, non-emergent healthcare service, experienced one of the largest decreases in volume among all modalities in radiology [2]. National organizations including the American College of Radiology, American Society of Breast Surgeons, and Society of Breast Imaging issued statements in March 2020 recommending that all screening mammography be postponed [3, 4]. Following these recommendations, many radiology practices saw a significant drop in the number of patients screened during late March–April 2020 [1], with some facilities reporting total screening volume drops of 99% [2].
In mid-April 2020, national organizations began to shift focus toward a cautious reopening of radiologic services, which included issuing recommendations that breast cancer screening services reopen using a phased approach based on patient personal risk for breast cancer [5, 6]. In May–July 2020, a slow recovery phase began as radiology practices gradually started offering services that had been temporarily halted [2, 7].
As patients returned to have their postponed screening mammograms, year-over-year comparisons of the volume of weekly mammograms performed emerged as a useful way to monitor the recovery of screening exam volumes to pre-pandemic levels [2, 8]. However, global metrics do not provide patient-level data which are important in determining individual patient populations that did and did not return for screening after having their mammogram postponed due to COVID-19. This information is of paramount importance in designing outreach efforts to encourage patients to resume breast cancer screening. Previous studies have suggested that several patient variables may affect patient breast cancer screening behaviors, including age [9–12], race [11–13], breast density [14], and socioeconomic variables [13, 15–17].
As society at large has slowly begun the process of recovery from the COVID-19 pandemic, the purpose of this study was to identify vulnerable patient populations with a lower likelihood of returning for delayed breast cancer screening who are at risk of being left behind by current recovery strategies. By identifying at-risk groups, targeted outreach can be extended to these patients as part of an inclusive and pro-active recovery effort toward resuming breast cancer screening.
Methods
This study was Institutional Review Board-approved and compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Mammographic screening examination volumes
The total number of screening mammograms performed at our institution by week was extracted from the electronic medical record for all of 2019 (baseline year) and all of 2020 (COVID-19-impacted year) through November 2020. Imaging was performed at the main breast care center, one of two satellite outpatient sites, or the mobile mammography bus. The numbers of screening mammograms performed in the baseline and COVID-19-impacted years were compared on a week-by-week and cumulative year-to-date basis.
Study population
Two patient cohorts were retrospectively created for the purposes of this study. The first cohort (COVID-19-impacted cohort) consisted of patients who received a screening mammogram from March 16, 2020, the date screening mammography had been stopped due to the pandemic, through October 31, 2020, the date our screening volumes and operations had been normalized to pre-pandemic volumes. The second cohort (baseline cohort) included patients who received a screening mammogram during the same time period in the preceding year (March 16, 2019 to October 31, 2019). These date ranges correspond with weeks 11 to 43 for both years. At our institution, screening mammograms scheduled from weeks 11–17 during the COVID-19-impacted year were postponed.
Clinical and demographic data
Demographic and clinical data were extracted from the medical record, including age, race, ethnicity, breast density, history of breast cancer, health insurance carrier, location of imaging facility used, and need for an interpreter for healthcare visits. For the baseline cohort, whether each patient experienced a “callback” from screening for additional diagnostic imaging of a suspicious finding in the baseline year was recorded as well as whether each patient returned for annual screening in the expected time window in the COVID-19-impacted year. For the purposes of this study, patients who presented for breast imaging in the expected annual screening window but who received diagnostic evaluation for breast symptoms at that encounter were included as having returned for breast cancer screening since, per our standard clinical practice, most of these patients received full screening examinations, as well, and were not expected to return again for imaging during that same annual screening window.
Geocoding
Patient postal addresses were geocoded to obtain the latitude, longitude, and census tract in which the patients reside using Topologically Integrated Geographic Encoding and Referencing (TIGER) files (2018) from the United States Census Bureau. Geocoding was performed using SAS software version 9.4 (SAS Institute, Cary, NC). Map visualizations were created in R (v4.0.2) using the TIGER files and the “sf” package.
Travel time
Travel time from each patient’s residence to the imaging center where the screening mammogram was performed was obtained through repeated calls to the Google Maps web page (Google, Menlo Park, CA) using the SAS FILENAME URL method in SAS version 9.4 [18]. Latitudes and longitudes of the patient addresses and each imaging site location were used to identify the shortest driving time by car without accounting for traffic. For patients with only a P.O. Box address listed, travel time was approximated from the centroid of the ZIP code of the P.O. Box to the imaging center. For patients imaged via a mobile mammography bus, travel time was calculated from the patient's residence to our primary imaging center.
Imputed socioeconomic variables
Census tract data were used in conjunction with the geocoded addresses to impute socioeconomic variables for each patient. Census tracts were linked to the American Community Survey 2015–2019 5-year estimates to get socioeconomic variables for each patient for whom we could identify a census tract. Specifically, the area-level median household income and percent of population living below the poverty line were obtained for each patient based on the data for the census tract in which they reside. Socioeconomic data could not be imputed for patients who had only a P.O. Box address listed or an address not recognized by the census data, such as those living in areas of new development.
Statistics
For the statistical analysis, patient clinical and demographic variables were dichotomized where possible and included age (less than 65 vs. greater than or equal to 65), self-identified race [categorized given the sample’s distribution by race as White vs. All Other Races (including Black, Asian, Native Hawaiian and Other Pacific Islander, Other Race, and Multiple Races)], ethnicity (non-Hispanic vs. Hispanic), breast density (non-dense vs. dense), health insurance status (any insurance carrier (including Medicare and Medicaid) vs. no insurance carrier/covered by charitable funds), type of imaging facility used (non-mobile imaging sites vs. mobile mammography bus), and 2019 screening mammogram BI-RADS Assessment Category [no callback (BI-RADS 1 or 2) vs. callback (BI-RADS 0)]. Drive time and imputed socioeconomic variables were not dichotomized. All other variables were dichotomized as yes/no.
Direct statistical comparison of the baseline and COVID-19-impacted cohorts was not possible due to partial overlap of the individuals in the two groups, but summary statistics were generated for comparison.
The baseline cohort was evaluated by univariate and multivariate logistic regression to identify patient variables associated with returning for breast imaging in the expected time window in the COVID-19-impacted year. In the subset of the baseline cohort patients who had imputed socioeconomic information, the univariate and multivariate logistic regression analyses were repeated with the socioeconomic information included as additional covariates. For these multivariate logistic regression analyses, a Wald test was conducted to determine whether the addition of the imputed socioeconomic variables improved the fit of the model.
Statistical analyses were performed using Stata version 14.2 (StataCorp, College Station, TX).
Results
Impact of COVID-19 on mammographic breast cancer screening volumes
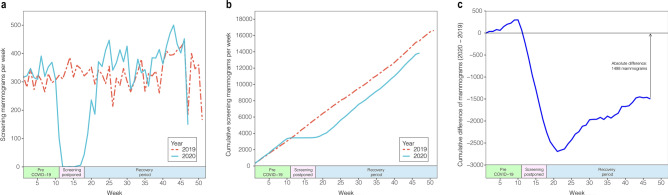
The number of screening mammograms performed during the COVID-19-impacted year was compared on a week-by-week basis with the number of screening mammograms performed during the baseline year (Fig. 1a). The expected drop in screening volumes was seen beginning in week 11, which corresponds with the onset of COVID-19-related closures. After gradual reopening of screening mammography began in week 18, an increase in volume occurred (Fig. 1a). Weekly screening volumes reached baseline levels at week 22 and remained higher than baseline until week 34.
Fig. 1.
Impact of COVID-19 on mammographic breast cancer screening volumes: a Screening mammograms performed by week for 2019 (baseline year) and 2020 (COVID-19-impacted year). b Cumulative year-to-date screening mammograms performed by week for 2019 and 2020. c Cumulative difference of year-to-date screening mammograms between 2019 and 2020
The cumulative year-to-date screening exam volumes were compared for the COVID-19-impacted and baseline years (Fig. 1b) and the cumulative difference between year-to-date screening mammograms was calculated (Fig. 1c). The difference in number of screening mammograms reached its greatest magnitude in week 21, with 2,962 fewer screening mammograms performed year-to-date in the COVID-19-impacted year compared to the baseline year. Starting with week 22, a significant recovery in the screening mammogram deficit was observed until week 43, at which point the deficit plateaued. At week 47, the cumulative total of screening mammograms performed was 15,339 in the baseline year and 13,841 in the COVID-19-impacted year, for a final cumulative deficit of 1,498 mammograms. This not only represents a 9.8% year-over-year decrease in cumulative screening mammograms but also a 49.4% reduction in the screening mammogram deficit from its maximum at week 22.
Comparison of post-COVID-19 and baseline cohorts
A total of 19,819 patients were included in this study, with 10,757 patients in the baseline cohort and 9062 patients in the COVID-19-impacted cohort. Due to a small amount of absent data in the medical record, sample sizes for the logistic regression analyses varied slightly based on available data, as indicated by the sample sizes noted in the tables.
While direct statistical comparison of the two cohorts was not possible due to expected overlap of the individuals in the groups, the COVID-19-impacted cohort had a slightly older median age, a relative increase in patients with dense breasts, and a higher fraction of patients with a history of breast cancer relative to the baseline cohort (Table 1). The COVID-19-impacted cohort had lower percentages of uninsured women and women imaged via the mobile mammography bus. Patients in the COVID-19-impacted cohort also had a shorter drive time relative to the baseline cohort (Table 1). Regarding the imputed socioeconomic variables, the COVID-19-impacted cohort had higher area-level median household income and lower area-level percent living below the poverty line than the baseline cohort (Table 1).
Table 1.
Comparison of the demographic, clinical, and imputed socioeconomic variables of the baseline (2019) and COVID-19-impacted (2020) cohorts
| Baseline cohort | COVID-19-impacted cohort | |
|---|---|---|
| Demographic and clinical variables | (n = 10,757) | (n = 9,062) |
| Age | ||
| Median [IQR] | 62 [54, 70] | 62 [53, 70] |
| Under 65 years of age | 57.3% | 58.2% |
| Race | ||
| White | 77.8% | 79.8% |
| Black | 13.8% | 14.5% |
| Asian or Pacific Islander | 1.4% | 1.2% |
| Other race or multiple races | 3.8% | 3.6% |
| Patient declined or race not recorded | 3.3% | 1.0% |
| Ethnicity | ||
| Non-Hispanic | 93.2% | 95.6% |
| Hispanic | 3.0% | 2.8% |
| Patient declined or ethnicity not recorded | 3.9% | 1.6% |
| Breast density (% non-dense) | 68.9% | 66.8% |
| Breast cancer history (% no history) | 90.3% | 89.2% |
| Insurance type (% Insured) | 97.5% | 98.0% |
| Imaging site type (% non-mobile) | 90.1% | 92.4% |
| Requires interpreter (% not requiring interpreter) | 97.1% | 97.4% |
| Travel time in minutes (median [IQR]) | 29 [15, 53] | 28 [15, 48] |
| Imputed socioeconomic variables | (n = 8,960) | (n = 7,637) |
| Median household income (median [IQR]) | $69,679 [$59,286, $80,828] | $71,272 [$60,200, $80,828] |
| Percent Under the Poverty Line (median [IQR]) | 8.7% [4.9%, 13.0%] | 8.4% [4.9%, 12.6%] |
Direct statistical comparison of the two cohorts was not possible due to partial overlap of the individuals in the two groups, but summary statistics were generated for comparison
IQR Inter-Quartile Range
Patient variables associated with returning for screening mammogram in the COVID-19-impacted year
Geographic analysis
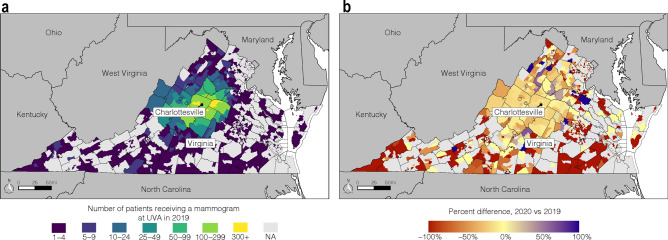
While the majority of patients in the baseline cohort came from regions in our primary catchment area, a significant proportion came from outlying regions (Fig. 2a). Patients from outlying region suffered the most dramatic percent decrease in returning for screening in the COVID-19-impacted year (Fig. 2b).
Fig. 2.
Impact of COVID-19 on mammographic breast cancer screening volumes by geographic region: a Number of patients screened at a University of Virginia mammography facility in 2019 by census tract. b Percent change in number of patients from a particular census tract screened at a University of Virginia facility in 2020 compared to 2019. Percent change calculated as [(# screened in 2020 − # screened in 2019)/# screened in 2019] × 100%
Univariate analysis
Univariate analysis of the baseline cohort demonstrated that the likelihood of returning for a screening mammogram in the COVID-19-impacted year was positively associated with older patient age [odds ratio (OR) 1.51; p < 001], dense breasts (OR 1.13; p = 0.004), having been called back from screening in 2019 for diagnostic workup (OR 1.28; p < 0.001), and personal history of breast cancer (OR 2.12; p < 0.001) (Table 2). Among patients with imputed socioeconomic data, the likelihood of returning in the COVID-19-impacted year was also positively associated with higher area-level median household income (OR 1.005; p < 0.001) (Table 3).
Table 2.
Univariate and multivariate logistic regression analyses of association between predictor variables and whether a patient in the baseline cohort (2019) returned for screening in the COVID-19-impacted year (2020) (n = 10,213 for multivariate model)
| Predictor variable | Univariate | p value | Multivariate | p value |
|---|---|---|---|---|
| OR [CI] | OR [CI] | |||
| Age | ||||
| Under 65 | – | – | – | – |
| 65 or older | 1.51 [1.39, 1.63] | < 0.001* | 1.29 [1.19, 1.41] | < 0.001* |
| Race | ||||
| White | – | – | – | – |
| All other races | 0.76 [0.69, 0.83] | < 0.001* | 0.88 [0.80, 0.98] | 0.021* |
| Ethnicity | ||||
| Non-Hispanic | – | – | – | – |
| Hispanic | 0.44 [0.37, 0.55] | < 0.001* | 0.77 [0.56, 1.05] | 0.104 |
| Breast density | ||||
| Non-dense | – | – | – | – |
| Dense | 1.13 [1.04, 1.23] | 0.004* | 1.10 [1.01, 1.21] | 0.027* |
| Insurance status | ||||
| Insured | – | – | – | – |
| Not insured | 0.42 [0.32, 0.55] | < 0.001* | 0.65 [0.47, 0.89] | 0.007* |
| Imaging site type | ||||
| Standard | – | – | – | – |
| Mobile | 0.14 [0.12, 0.17] | < 0.001* | 0.27 [0.22, 0.32] | < 0.001* |
| Called back from screening in 2019 | ||||
| Yes | – | – | – | – |
| No | 1.28 [1.12, 1.46] | < 0.001* | 1.13 [0.98, 1.30] | 0.104 |
| History of breast cancer | ||||
| No | – | – | – | – |
| Yes | 2.12 [1.84, 2.44] | < 0.001* | 1.78 [1.54, 2.06] | < 0.001* |
| Requires interpreter | ||||
| No | – | – | – | – |
| Yes | 0.41 [.32, 0.52] | < 0.001* | 0.68 [0.48, 0.96] | 0.029* |
| Travel time to imaging center† (continuous) | 0.77 [0.73, 0.80] | < 0.001* | 0.88 [0.84, 0.91] | < 0.001* |
“All Other Races” includes Black, Asian or Pacific Islander, Other Race or Multiple Races, and Patient Declined or Race Not Recorded
OR odds ratio, CI 95% confidence interval
*Statistically significant (p ≤ 0.05)
†In hours
Table 3.
Univariate regression analyses testing the association between imputed socioeconomic predictor variables and whether a baseline cohort (2019) patient returned for screening in the COIVD-19-impacted year (2020)
| Predictor variable | Univariate | |
|---|---|---|
| OR [CI] | p value | |
| Median household income† (Continuous) | 1.005 [1.003, 1.007] | < 0.001* |
| Percent living below poverty level (continuous) | 0.982 [0.976, 0.987] | < 0.001* |
OR odds ratio, CI 95% confidence interval
*Statistically significant (p ≤ 0.05)
‡In thousands of dollars
Univariate analysis of the COVID-19-impacted cohort also demonstrated that the likelihood of returning for a screening mammogram was negatively associated with numerous variables including self-reported race other than White (OR 0.76; p < 0.001), self-reported Hispanic ethnicity (OR 0.44; p < 0.001), uninsured status (OR 0.42; p < 0.001), being imaged via the mobile mammography bus (OR 0.14; p < 0.001), requiring an interpreter (OR 0.41; p < 0.001), and higher travel time from home to imaging center (OR 0.77; p < 0.001) (Table 2). Among those with imputed socioeconomic data, the likelihood of returning for a screening mammogram in the COVID-19-impacted year was also negatively associated with higher area-level percent living below the poverty line (OR 0.982; p < 0.001) (Table 3).
Multivariate analysis
Multivariate analysis demonstrated an independent positive association between returning in the COVID-19-impacted year for screening and older patient age (OR 1.29; p < 001), dense breasts (OR 1.10; p = 0.027), and personal history of breast cancer (OR 1.78; p < 0.001) (Table 2). There was also an independent negative association between returning in the COVID-19-impacted year for screening and self-reported race other than White (OR 0.88; p = 0.021), uninsured status (OR 0.65; p = 0.007), being imaged on the mobile mammography bus (OR 0.27; p < 0.001), requiring an interpreter (OR 0.68; p = 0.029), and longer travel time to imaging center (OR 0.88; p < 0.001). The variables of ethnicity and being called back from screening in the baseline year were not independently associated with returning for screening in the COVID-19-impacted year (Table 2).
Influence of socioeconomic factors
Adding the imputed socioeconomic variables (median household income and percent living below the poverty line) improved the fit of our multivariate regression models (p = 0.048) (Table S1). This improved fit appears to be due primarily to the variable of percent living below the poverty line, which demonstrated an independent association with returning for screening in the COVID-19-impacted year (OR 0.991; p = 0.014) (Table S1). Interestingly, the addition of the imputed socioeconomic variables led to race dropping out of statistical significance (OR 0.90; p = 0.067) (Table S1).
Discussion
The purpose of this study was to identify patient populations at risk for delayed breast cancer screening due to COVID-19 in order to facilitate inclusive, targeted outreach to these patient populations. Such outreach is essential to prevent these patients from suffering the consequences of delayed screening, including delayed diagnosis and later-stage presentation of breast cancer.
Our screening mammogram volume data demonstrate that after gradual reopening of screening mammography began in week 18 of the COVID-19-impacted year, an increase in screening volumes reduced the deficit in cumulative screening mammograms compared to the baseline year, but only by 49.4% as of week 47. This suggests that up to half of patients whose screening mammograms were delayed due to COVID-19 still had not returned for their screening mammogram as of late 2020 and are at risk for being left behind by current COVID-19 recovery strategies if they are not updated in a way that helps engage these patients.
Our study demonstrated an independent association between younger patient age and a lower likelihood of returning for breast cancer screening in the COVID-19-impacted year. This reluctance of younger patients to return for screening was unexpected, given the lower risk for younger patients of severe complications associated with COVID-19 infection [19–21] and the reported lower concern about COVID-19 among individuals under 65 years of age [22]. The lower likelihood of younger patients to return for screening could reflect a higher concern about COVID-19 among younger patients than previously realized or possibly a lower concern about breast cancer relative to older patients. Other patient factors, such as employment or family responsibilities, may also have influenced whether younger patients returned for screening.
Including the imputed socioeconomic variables of median household income and percent living below the poverty line in our logistic regression analysis improved the fit of our model. In particular, there was an independent association between patients residing in higher poverty areas and a lower likelihood of returning for screening in the COVID-19-impacted year. These findings are consistent with other studies which have shown that patients from disadvantaged areas are less likely to receive recommended mammographic screening than women living in the most advantaged areas [15, 17]. Our study suggests that this trend has been exacerbated by the COVID-19 pandemic. Census tract data are readily and publicly available and could be used to determine which patients would be at risk based on area-level socioeconomic variables.
Interestingly, while our initial regression analysis suggested that self-reported non-White race was associated with a lower likelihood of returning for screening, this association fell out of statistical significance when socioeconomic variables were added to the model. This suggests that the association of race with the likelihood of returning for screening in the COVID-19-impacted year is actually due to an association of race with the socioeconomic variables. This finding is consistent with other studies which have demonstrated that associations between race and breast cancer screening are attenuated or eliminated when accounting for socioeconomic variables [23–25]. However, several studies have also demonstrated continued associations between Black race and larger tumor size and later-stage breast cancer at diagnosis [26, 27] and lower access in general to cancer screening and early diagnosis [28], highlighting the health disparities which continue to persist and the role that race continues to play in breast cancer screening and diagnosis. As healthcare systems recover from the effects of COVID-19, every effort must be made to actively engage and reach out to such at-risk groups to prevent exacerbation of pre-existing disparities in healthcare.
Not surprisingly, we found that the lack of health insurance and the need for an interpreter were independently associated with a lower likelihood of returning for screening in the COVID-19-impacted year. Previous studies have reported that a lack of health insurance [11, 29] and limited English proficiency [30] are deterrents to obtaining mammographic breast cancer screening. Our data suggest that the effects of the economic and social obstacles created by a lack of insurance and by a need for interpreter services have worsened during the COVID-19 pandemic, leading to an increased rate of not returning for screening in the COVID-19-impacted year for these individuals.
Our data demonstrate that patients who live at greater distances from the imaging center were less likely to return for screening in the COVID-19-impacted year. The literature is mixed with respect to the effect of geographic accessibility to mammographic breast cancer screening [31], but much of this heterogeneity may be due to differences in how geographic accessibility was measured. While prior studies have often used imaging center density [32, 33] or distances between ZIP codes [34] to determine how far a patient would have to travel for screening, we instead calculated actual drive times using a combination of geocoding and an online map service. This allowed us to include in our models a personalized, real-world measurement of geographic accessibility to mammographic screening for each patient.
Women who were imaged by the mobile mammography bus were less likely to return in the COVID-19-impacted year even though our mammography bus service reopened within one month of the reopening of our traditional imaging centers. The trend remained statistically significant when controlling for travel time. This suggests that the population that uses mobile mammography services may have concerns beyond just those of distance—for example, they may not have readily accessible transportation, they might live in areas with different local concerns, and/or social attitudes toward healthcare, etc.
As expected, we found that patients with no history of breast cancer were less likely to return for screening in the COVID-19-impacted year than those who had a history of breast cancer. Interestingly, we also found an independent association between non-dense breast tissue and not returning for screening. This may be due to the fact that at our institution we have historically been very active in educating patients and providers about the increased risk for breast cancer for women with dense breasts, so it is not unexpected that our local patient population of women with dense breasts would be more likely to return for screening than patients with non-dense breasts.
Consistent with other studies [35–37], we also found that a history of being called back from a screening exam for additional diagnostic workup in the baseline year was not associated with whether a patient returned for screening in the COVID-19-impacted year.
Our study has limitations. This is a single institution study and therefore the findings may not be generalizable to other patient populations, including populations with higher levels of racial, ethnic, or socioeconomic diversity. Our socioeconomic data were imputed from census tract data since patient-provided individual socioeconomic data were not available. In addition, imputation of socioeconomic variables was not possible for all patients, including patients without a postal address listed in the medical record or with an address not recognized by the census data, such as those living in areas of new development. This could bias our results if a particular group of patients was either over- or under-represented in the cohort of patients for whom socioeconomic data could not be imputed.
In conclusion, our study identified several patient variables that are independently associated with a lower likelihood of returning for screening after COVID-19 closures, including younger patient age, residing in an area with a higher level of poverty, lack of health insurance, need for an interpreter, longer travel time to imaging center, utilization of mobile mammography services, no history of breast cancer, and non-dense breast tissue. Targeted outreach to patients in these populations, who are at risk of being left behind by current COVID-19 recovery strategies, may be an effective means of helping them resume recommended breast cancer screening and other needed medical care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Rajesh Desai, MA, research specialist and lead programmer for the Population Health and Cancer Outcomes Core, UVA Cancer Center, for his assistance with geocoding addresses, linking U.S. Census data to obtain socioeconomic information, and calculating patient travel times.
Abbreviations
- IQR
Interquartile range
- OR
Odds ratio
- CI
Confidence interval
Funding
Not applicable.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
None of the authors has any conflict of interest or competing interests related to this study.
Ethical approval
This retrospective study was Institutional Review Board-approved and compliant with HIPAA.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malhotra A, Wu X, Fleishon HB, Duszak R, Jr, Silva E, 3rd, McGinty GB, Bender C, Williams B, Pashley N, Stengel CJB, Naidich JJ, Hughes D, Sanelli PC. Initial impact of COVID-19 on radiology practices: An ACR/RBMA survey. J Am Coll Radiol. 2020;17(11):1525–1531. doi: 10.1016/j.jacr.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norbash AM, Moore AV, Jr, Recht MP, Brink JA, Hess CP, Won JJ, Jain S, Sun X, Brown M, Enzmann D. Early-stage radiology volume effects and considerations with the coronavirus disease 2019 (COVID-19) Pandemic: adaptations, risks, and lessons learned. J Am Coll Radiol. 2020;17(9):1086–1095. doi: 10.1016/j.jacr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ASBrS and ACR Joint Statement on Breast Screening Exams During the COVID-19 Pandemic (2020). https://www.breastsurgeons.org/news/?id=45. Accessed 10 Feb 2021
- 4.Society of Breast Imaging Statement on Breast Imaging during the COVID-19 Pandemic (2020). https://www.sbi-online.org/Portals/0/Position%20Statements/2020/society-of-breast-imaging-statement-on-breast-imaging-during-COVID19-pandemic.pdf. Accessed 10 Feb 2021
- 5.ACR Guidelines for Resumption of Breast Cancer Screening (2020) https://www.acr.org/-/media/ACR/Files/Breast-Imaging-Resources/Care-Toolkit/ACR-Guidelines-for-Resumption-of-Breast-Cancer-Screening.pdf. Accessed 10 Feb 2021
- 6.SBI Recommendations for a Thoughtful Return to Caring for Patients (2020) https://www.sbi-online.org/Portals/0/Position%20Statements/2020/SBI-recommendations-for-a-thoughtful-return-to-caring-for-patients_April-16-2020.pdf. Accessed 10 Feb 2021
- 7.Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin N Am. 2021;59(1):1–11. doi: 10.1016/j.rcl.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H, Bergman A, Chen AT, Ellis D, David G, Friedman AB, Bond AM, Bailey JM, Brooks R, Smith-McLallen A. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2020 doi: 10.1111/1475-6773.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MM, Repich K, Patrie JT, Anderson RT, Harvey JA. Patient characteristics associated with patient-reported deterrents to adjunct breast cancer screening among patients with dense breasts. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.24516. [DOI] [PubMed] [Google Scholar]
- 10.Yuan C, Kulkarni K, Dashevsky BZ. Preventive care: how mammography utilization changes as women age. J Am Coll Radiol. 2020;17(2):238–247. doi: 10.1016/j.jacr.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Davis J, Liang J, Petterson MB, Roh AT, Chundu N, Kang P, Matz SL, Connell MJ, Gridley DG. Risk factors for late screening mammography. Curr Probl Diagn Radiol. 2019;48(1):40–44. doi: 10.1067/j.cpradiol.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Bynum JP, Braunstein JB, Sharkey P, Haddad K, Wu AW. The influence of health status, age, and race on screening mammography in elderly women. Arch Intern Med. 2005;165(18):2083–2088. doi: 10.1001/archinte.165.18.2083. [DOI] [PubMed] [Google Scholar]
- 13.Elewonibi BR, Thierry AD, Miranda PY. Examining mammography use by breast cancer risk, race, nativity, and socioeconomic status. J Immigr Minor Health. 2018;20(1):59–65. doi: 10.1007/s10903-016-0502-3. [DOI] [PubMed] [Google Scholar]
- 14.Santiago-Rivas M, Benjamin S, Andrews JZ, Jandorf L. breast density awareness and knowledge, and intentions for breast cancer screening in a diverse sample of women age eligible for mammography. J Cancer Educ. 2019;34(1):90–97. doi: 10.1007/s13187-017-1271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calo WA, Vernon SW, Lairson DR, Linder SH. Area-level socioeconomic inequalities in the use of mammography screening: a multilevel analysis of the health of Houston survey. Womens Health Issues. 2016;26(2):201–207. doi: 10.1016/j.whi.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Jang SN. Socioeconomic disparities in breast cancer screening among US women: trends from 2000 to 2005. J Prev Med Public Health. 2008;41(3):186–194. doi: 10.3961/jpmph.2008.41.3.186. [DOI] [PubMed] [Google Scholar]
- 17.Dailey AB, Brumback BA, Livingston MD, Jones BA, Curbow BA, Xu X. Area-level socioeconomic position and repeat mammography screening use: results from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2331–2344. doi: 10.1158/1055-9965.EPI-11-0528. [DOI] [PubMed] [Google Scholar]
- 18.Zdeb M (2010) Driving distances and drive times using SAS and Google Maps. https://support.sas.com/resources/papers/proceedings10/050-2010.pdf. . Accessed 22 Feb 2021
- 19.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czeisler ME, Tynan MA, Howard ME, Honeycutt S, Fulmer EB, Kidder DP, Robbins R, Barger LK, Facer-Childs ER, Baldwin G, Rajaratnam SMW, Czeisler CA. Public attitudes, behaviors, and beliefs related to COVID-19, stay-at-home orders, nonessential business closures, and public health guidance—United States, New York City, and Los Angeles, May 5–12, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):751–758. doi: 10.15585/mmwr.mm6924e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko NY, Hong S, Winn RA, Calip GS. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol. 2020;6(3):385–392. doi: 10.1001/jamaoncol.2019.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman LA. Breast cancer disparities: socioeconomic factors versus biology. Ann Surg Oncol. 2017;24(10):2869–2875. doi: 10.1245/s10434-017-5977-1. [DOI] [PubMed] [Google Scholar]
- 25.Du XL, Fang S, Meyer TE. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol. 2008;31(2):125–132. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- 26.Williams F, Thompson E. Disparities in breast cancer stage at diagnosis: importance of race, poverty, and age. J Health Dispar Res Pract. 2017;10(3):34–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy D, Du DY. Socioeconomic and racial disparities in cancer stage at diagnosis, tumor size, and clinical outcomes in a large cohort of women with breast cancer, 2007–2016. J Racial Ethn Health Disparities. 2020 doi: 10.1007/s40615-020-00855-y. [DOI] [PubMed] [Google Scholar]
- 28.Scally BJ, Krieger N, Chen JT. Racialized economic segregation and stage at diagnosis of colorectal cancer in the United States. Cancer Cause Control. 2018;29(6):527–537. doi: 10.1007/s10552-018-1027-y. [DOI] [PubMed] [Google Scholar]
- 29.Hsia J, Kemper E, Kiefe C, Zapka J, Sofaer S, Pettinger M, Bowen D, Limacher M, Lillington L, Mason E, Investigato WHI. The importance of health insurance as a determinant of cancer screening: evidence from the Women’s Health Initiative. Prev Med. 2000;31(3):261–270. doi: 10.1006/pmed.2000.0697. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs EA, Karavolos K, Rathouz PJ, Ferris TG, Powell LH. Limited English proficiency and breast and cervical cancer screening in a multiethnic population. Am J Public Health. 2005;95(8):1410–1416. doi: 10.2105/AJPH.2004.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan-Gates JA, Ersek JL, Eberth JM, Adams SA, Pruitt SL. Geographic access to mammography and its relationship to breast cancer screening and stage at diagnosis: a systematic review. Womens Health Issues. 2015;25(5):482–493. doi: 10.1016/j.whi.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkin EB, Ishill NM, Snow JG, Panageas KS, Bach PB, Liberman L, Wang FH, Schrag D. Geographic access and the use of screening mammography. Med Care. 2010;48(4):349–356. doi: 10.1097/MLR.0b013e3181ca3ecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coughlin SS, Leadbetter S, Richards T. Sabatino SA (2008) Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women. Soc Sci Med. 2002;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Engelman KK, Hawley DB, Gazaway R, Mosier MC, Ahluwalia JS, Ellerbeck EF. Impact of geographic barriers on the utilization of mammograms by older rural women. J Am Geriatr Soc. 2002;50(1):62–68. doi: 10.1046/j.1532-5415.2002.50009.x. [DOI] [PubMed] [Google Scholar]
- 35.Taksler GB, Keating NL, Rothberg MB. Implications of false-positive results for future cancer screenings. Cancer. 2018;124(11):2390–2398. doi: 10.1002/cncr.31271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinckney RG, Geller BM, Burman M, Littenberg B. Effect of false-positive mammograms on return for subsequent screening mammography. Am J Med. 2003;114(2):120–125. doi: 10.1016/s0002-9343(02)01438-9. [DOI] [PubMed] [Google Scholar]
- 37.Hardesty LA, Lind KE, Gutierrez EJ. Compliance with screening mammography guidelines after a false-positive mammogram. J Am Coll Radiol. 2016;13(9):1032–1038. doi: 10.1016/j.jacr.2016.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.