Abstract
Background: Life expectancy is increasing along with the rising prevalence of cognitive disorders. Among the factors that may contribute to their prevalence, modifiable risk factors such as diet may be of primary importance. Unarguably, plant-based diets rich in bioactive compounds, such as polyphenols, showed their potential in decreasing risk of neurodegenerative disorders. Therefore, the aim of the present study is to investigate whether exposure to components of plant-based diets, namely phenolic acids, may affect cognitive status in older Italian adults. Methods: The demographic, lifestyle and dietary habits of a sample of individuals living in southern Italy were analyzed. Dietary intake was assessed through food frequency questionnaires (FFQs). Data on the phenolic acids content in foods were estimated using the Phenol-Explorer database. Cognitive status was evaluated using The Short Portable Mental Status Questionnaire. Multivariate logistic regression analyses were used to assess the associations. Results: The mean intake of phenolic acids was 346.6 mg/d. After adjustment for potential confounding factors, individuals in the highest quartile of total phenolic acid intake were less likely to have impaired cognitive status (OR = 0.36 (95% CI: 0.14, 0.92)); similarly, the analysis for subclasses of phenolic acids showed the beneficial effect toward cognitive status of greater intake of hydroxycinnamic acids (OR = 0.35 (95% CI: 0.13, 0.91)). Among individual compounds, only higher intake of caffeic acid was inversely associated with impaired cognitive status (OR = 0.32 (95% CI: 0.11, 0.93)); notably, the association with ferulic acid intake was significant only when adjusting for background characteristics, and not for adherence to the Mediterranean diet. Conclusions: This study revealed that greater intakes of dietary phenolic acids were significantly inversely associated with impaired cognition, emphasizing the possible role of phenolic acids in the prevention of cognitive disorders.
Keywords: phenolic acids, hydroxycinnamic acids, hydroxybenzoic acids, ferulic acid, caffeic acid, cognitive status, neuroinflammation, coffee, tea, cohort
1. Introduction
Dietary patterns characterized by a high content in plant-based foods are considered a gold standard to maintain health and reduce the risk of non-communicable diseases [1,2]. The main features of a healthy diet include consumption of fruit and vegetables, nuts, and wholegrains as major food groups exerting positive effects on human health [3,4,5]. In fact, current evidence has underlined the importance of their content in fiber, vitamins and non-vitamin antioxidants in contributing to the health-promoting effects of plant-based dietary patterns [6]. In addition, emphasis has been focused on plant-based beverages, such as tea and coffee, which are consumed worldwide and have demonstrated peculiar benefits for disease prevention [7,8]. A common characteristic of the aforementioned food and beverage groups is the relatively high content in polyphenols. These phytochemicals are widespread in nature and have a number of beneficial effects for plants and as more, were demonstrated to exert positive effects for humans as well. Phytochemicals have been the focus of recent research further supporting the health benefits of plant-based foods and beverages [9].
Observational studies conducted on humans exploring the health benefits of phenolic acids are limited, as research focus mostly on flavonoids and their potential capacity to reduce the risk of hypertension [10], type-2 diabetes [11], and cardiovascular diseases (CVD) [12,13]. Phenolic acids are compounds containing a phenolic ring and an organic carboxylic acid function (C6–C1 skeleton). Phenolic acids are classified as hydroxybenzoic acids (which include individual compounds such as gallic, vanillic, protocatechuic, syringic and salicylic acids) and hydroxycinnamic acids (which include cinnamic, p-coumaric, ferulic, rosmarinic, caffeic and chlorogenic acid). The major dietary sources of phenolic acids include fruits, wholegrains and nuts, cocoa as well as beverages such as coffee and beer [14]. Findings from observational studies on phenolic acid intake have shown an inverse association of their consumption with hypertension [15], type-2 diabetes [16], metabolic syndrome [17], and non-alcoholic fatty liver disease [18]. However, data on outcomes other than cardio-metabolic diseases is quite limited. A plausible effect of total phenolic acids and hydroxycinnamic acids intake was found toward depressive symptoms [19] and sleep quality [20], respectively. Regarding cognitive health, only two studies explored the association between phenolic acid intake and cognitive status; nonetheless, one of the studies provided estimates for total phenolic acid intake, while the other one only for hydroxybenzoic acids. Therefore, the aim of this study was to provide a comprehensive view on the association between dietary phenolic acid intake and cognitive status, considering not only their total intake but also the effect of individual compounds in a cohort of older Italian adults.
2. Materials and Methods
2.1. Study Population
The MEAL study is an observational study focusing on the link between nutritional and lifestyle habits typical to the Mediterranean area and non-communicable diseases. The study protocol has been described elsewhere [21]. Briefly, the study enrollment took place between 2014 and 2015 in the main districts of the city of Catania in southern Italy. A sample of 2044 men and women aged 18 or more years old was randomly selected and included in the baseline data. Data collection was carried out on using the registered records of local general practitioners stratified by sex and 10-year age groups. In order to provide a specific relative precision of 5% (Type I error, 0.05; Type II error, 0.10), considering an anticipated 70% participation rate, the theoretical sample size was estimated to 1500 individuals.
Out of 2405 individuals invited to participate in the study, 361 refused leaving 2044 participants (response rate of 85%) as the final sample. Taking into consideration that the investigated outcome is more prevalent in the older population, the analysis for the present study was limited to individuals 50 years old or older (n = 916). All participants were familiarized about goals of the MEAL study and informed written consent was obtained. The study protocol has been refereed and approved by the concerning ethical committee and all the study procedures were conducted in accordance with the Declaration of Helsinki (1989) of the World Medical Association.
2.2. Data Collection
A face-to-face assisted personal interview was conducted during which all participants were provided with a paper copy of the questionnaire in order to visualize the response options. However, the final answers were registered directly by the interviewer using tablet computers. Information regarding sociodemographic and lifestyle factors was collected [22]. The sociodemographic data included age at recruitment, sex, and educational status (low (primary/secondary school), medium (high school), and high (university)). Lifestyle variables included physical activity (low, moderate, high; determined by using the International Physical Activity Questionnaire (IPAQ) [23]), smoking status (non-smoker, ex-smoker, and current smoker), and alcohol drinking (none, moderate drinker (0.1–12 g/d) and regular drinker (>12 g/d)).
2.3. Dietary Assessment
Aiming to determine the dietary intake, two food frequency questionnaires (FFQ, a long and a short version) previously proved for validity and reliability for the Sicilian population were adapted [24,25]. Employing the determination of the food intake, the energy content as well as the macro- and micronutrients intake was calculated using food composition tables of the Italian Research Center for Foods and Nutrition [26]. Intake of seasonal foods referred to consumption during the period in which the food was available adjusting by its proportional intake over one year.
Diet quality was evaluated by means of adherence to the Mediterranean diet [27]. Briefly, a literature-based scoring system, which is based on the frequency of intake of different food groups was used. In particular, food groups characteristic to the Mediterranean dietary pattern (such as fruit, vegetables, cereals, legumes, fish, and olive oil) were given positive points, while food groups not representing it (such as meat and dairy products) were given negative points; moderate alcohol intake was deemed as optimal for higher adherence. The final score indicative of adherence to the Mediterranean diet was based on nine food categories with a score ranging from 0 (lowest adherence) to 18 points (highest adherence), and individuals clustered in tertiles categorized as low, medium, and high adherent to the Mediterranean diet [28]. FFQs with unreliable intakes (<1000 or >6000 kcal/d) were excluded from the analyses (n = 33) leaving a total of 883 individuals included in the analysis.
2.4. Estimation of Phenolic Acid Consumption
The process of the estimation of dietary phenolic acid intake has been previously described in detail [29]. Briefly, data on the phenolic acid content in plant-based foods and beverages were retrieved from the Phenol-Explorer database [14]. First, the food and beverage consumption was calculated (in g or mL) by following the standard portion sizes used in the study and then converted in 24-h intake. Afterwards, the Phenol-Explorer database was searched to retrieve mean content values for all phenolic acids contained in the applicable food items included in the FFQ, and phenolic acid intake from each food was determined by multiplying the content of total, main subclasses, and selected individual phenolic acids by the daily consumption of each food adjusted for total energy intake (kcal/d) using the residual method [30].
2.5. Cognitive Status Evaluation
Cognitive status assessment in the MEAL study was performed using the Short Portable Mental Status Questionnaire (SPMSQ) [31], which was designed to measure cognitive impairment in both the general and hospital population [32], and previously applied in the Italian population [33]. SPMSQ, a 10-item tool, was administered by the clinician in the office or in a hospital. The predefined domains for assessment of the screening tool were the following: (i) intact, less than 3 errors; (ii) mild, 3–4 errors; (iii) moderate, 5–7 errors, and (iv) severe, 8 or more errors. For this study, we considered more than 2 errors as a cut-off point for impaired cognitive status.
2.6. Statistical Analysis
Categorical variables are presented as frequencies of occurrence and percentages; differences between groups were tested with Chi-squared test. Continuous variables are presented as means and standard deviations (SDs) or median and standard errors (SEs) whether they were distributed normally or skewed, respectively; differences between groups were tested with Student’s t-test or Mann–Whitney U-test for variables distributed normally or skewed, respectively. The relation between exposure variables and cognitive status was tested through uni- and multivariate logistic regression analysis adjusted for baseline characteristics (age, sex, educational status, smoking and alcohol drinking habits, and physical activity level). An additional model was performed to further adjust for adherence to the Mediterranean diet, as a proxy variable for diet quality. The association between major dietary sources of phenolic acids and cognitive status was finally evaluated. All reported p-values were based on two-sided tests and compared to a significance level of 5%. SPSS 17 (SPSS Inc., Chicago, IL, USA) software was used for all the statistical calculations.
3. Results
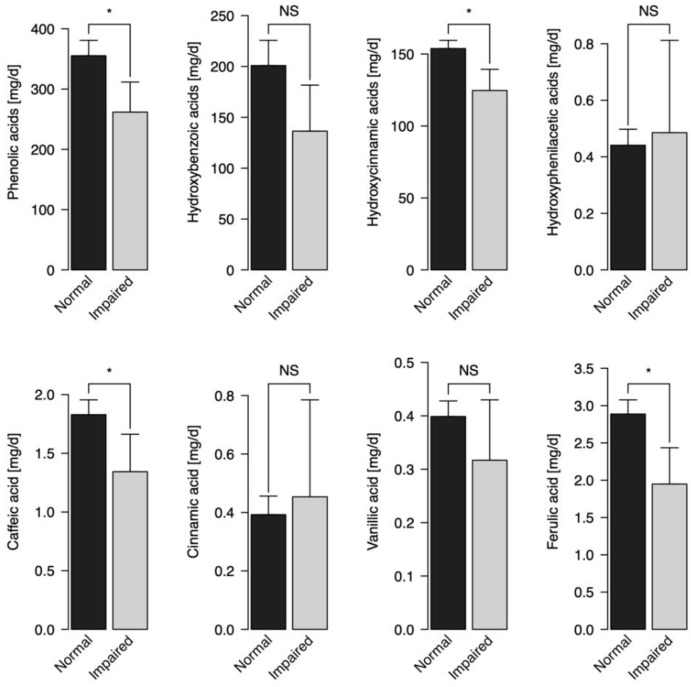
A total of 883 individuals were included in the analyses of this study. The mean age was 64.9 years old, 56.7% were female. Eighty individuals (9.1% of the sample) were categorized as having impaired cognitive status. The mean intake of phenolic acids was 346.6 mg/d. Comparison of the mean intake of total, subgroups, and individual phenolic acids by cognitive status is reported in Figure 1.
Figure 1.
Comparison of the mean intake of total, subgroups, and individual phenolic acids by cognitive status. Barplots represent mean values of phenolic acids intake, while whiskers represent upper bounds of 95% confidence intervals for mean values of phenolic acids intake. NS—not significant; * p < 0.05.
The mean intake of all the types of phenolic acids was significantly higher in individuals with normal cognitive status when comparing with individuals with impaired cognitive status (Figure 1): total phenolic acids (355.3 mg/d (95% CI: 329.9, 380.69) vs. 261.8 mg/d (95% CI: 212.04, 311.57)), hydroxycinnamic acids (153.77 mg/d (95% CI: 148.1, 159.43) vs. 124.62 mg/d (95% CI: 109.9, 139.34)), caffeic acid (1.83 mg/d (95% CI: 1.7–1.95) vs. 1.34 mg/d (95% CI:1.02, 1.66)) and ferulic acid (2.89 mg/d (95% CI: 2.7, 3.08) vs. 1.95 mg/d (95% CI: 1.46, 2.44)).
To test whether intake of phenolic acid was distributed differently across potential confounding factors, the main background characteristics of the study sample categorized by quartiles of phenolic acid intake are presented in Table 1. The age of study participants varied when considering level of the exposure to dietary phenolic acid intake. There were significant differences in the distribution of the variables such as smoking status and physical activity level among the quartiles of phenolic acids intake, however with no particular trends toward healthier or unhealthier behaviors. Additionally, there was a higher proportion of regular alcohol drinkers among the highest quartile of phenolic acid intake. When considering the quartiles with greater phenolic acid intake, a higher proportion of individuals with high adherence to the Mediterranean diet was observed (Table 1).
Table 1.
Background characteristics by quartiles of dietary phenolic acid intake in the study sample (n = 883).
| Phenolic Acid Intake | |||||
|---|---|---|---|---|---|
| Q1, n = 219 (Mean = 114.4 mg/d) |
Q2, n = 220 (Mean = 202.7 mg/d) |
Q3, n = 208 (Mean = 306.6 mg/d) |
Q4, n = 236 (Mean = 711.8 mg/d) |
p-Value | |
| Age groups, mean (SD) | 67.1 (10.3) | 65.6 (9.8) | 63.1 (8.2) | 64.1 (9.5) | <0.001 |
| Sex, n (%) | 0.053 | ||||
| Men | 46 (33.6) | 67 (46.2) | 49 (33.8) | 63 (43.4) | |
| Women | 91 (66.4) | 78 (53.8) | 96 (66.2) | 82 (56.6) | |
| Educational status, n (%) | 0.812 | ||||
| Low | 88 (64.2) | 86 (59.3) | 80 (55.2) | 88 (60.7) | |
| Medium | 29 (21.2) | 38 (26.2) | 41 (28.3) | 34 (23.4) | |
| High | 20 (14.6) | 21 (14.5) | 24 (16.6) | 23 (15.9) | |
| Smoking status, n (%) | 0.001 | ||||
| Never smoker | 84 (61.3) | 76 (52.4) | 93 (64.1) | 87 (60.0) | |
| Former smoker | 15 (10.9) | 39 (26.9) | 35 (24.1) | 29 (15.2) | |
| Current smoker | 38 (27.7) | 30 (20.7) | 17 (11.7) | 36 (24.8) | |
| Physical activity level, n (%) | <0.001 | ||||
| Low | 45 (41.3) | 39 (32.0) | 41 (33.9) | 21 (18.8) | |
| Moderate | 34 (31.2) | 63 (51.6) | 71 (58.7) | 70 (62.5) | |
| High | 30 (27.5) | 20 (16.4) | 9 (7.4) | 21 (18.8) | |
| Alcohol intake, n (%) | <0.001 | ||||
| No | 28 (20.4) | 46 (31.7) | 40 (27.6) | 25 (17.2) | |
| Moderate | 96 (70.1) | 78 (53.8) | 66 (45.5) | 61 (42.1) | |
| Regular | 13 (9.5) | 21 (14.5) | 39 (26.9) | 59 (40.7) | |
| Mediterranean diet adherence, n (%) | <0.001 | ||||
| Low | 54 (27.7) | 55 (24.6) | 26 (11.5) | 33 (13.9) | |
| Medium | 64 (32.8) | 63 (28.1) | 65 (28.6) | 65 (27.4) | |
| High | 77 (39.5) | 106 (47.3) | 136 (59.9) | 139 (58.6) | |
After adjustment for potential confounding factors, individuals in the highest quartile of total phenolic acid intake (median intake = 509.2 mg/day) were less likely to have impaired cognitive status than those consuming the least (OR = 0.36 (95% CI: 0.14, 0.92)), although there was no clear trend across quartiles of exposure (Table 2). The analysis for subclasses of phenolic acids showed that individuals with the highest intake of hydroxycinnamic acids (highest vs. lowest category, OR = 0.35 (95% CI: 0.13, 0.91)) were less likely to have impaired cognitive status (Table 2). Moreover, a similar inverse association was also found for the third versus the first quartile of intake of hydroxyphenylacetic acid (OR = 0.26 (95% CI: 0.11, 0.63)). Among individual compounds investigated, only higher intake of caffeic acid resulted inversely associated with impaired cognitive status (OR = 0.32 (95% CI: 0.11, 0.93)); notably, the association with ferulic acid intake was significant when adjusting for background characteristics, but further adjustment for adherence to the Mediterranean diet nullified the results (OR = 0.42 (95% CI: 0.16, 1.07)).
Table 2.
Association between total, main classes, and individual phenolic acid intake and impaired cognitive status.
| Phenolic Acid Intake | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Phenolic acids, median (SE), mg/day | 122.1 (2.4) | 205.2 (1.8) | 302.9 (2.5) | 509.2 (34.0) |
| Model 1, OR (95% CI) a | 1 | 0.33 (0.17–0.64) | 0.51 (0.28–0.94) | 0.34 (0.17–0.71) |
| Model 2, OR (95% CI) b | 1 | 0.26 (0.12–0.57) | 0.69 (0.32–1.45) | 0.35 (0.14–0.89) |
| Model 3, OR (95% CI) c | 1 | 0.27 (0.13–0.58) | 0.74 (0.34–1.57) | 0.36 (0.14–0.92) |
| Hydroxybenzoic acids, median (SE), mg/day | 8.8 (0.8) | 64.0 (0.5) | 131.6 (2.1) | 284.2 (34.4) |
| Model 1, OR (95% CI) a | 1 | 0.83 (0.45–1.53) | 0.77 (0.40–1.48) | 0.73 (0.38–1.41) |
| Model 2, OR (95% CI) b | 1 | 0.84 (0.40–1.74) | 0.82 (0.38–1.77) | 1.01 (0.45–2.22) |
| Model 3, OR (95% CI) c | 1 | 0.84 (0.40–1.75) | 0.82 (0.38–1.78) | 1.00 (0.45–2.22) |
| Hydroxycinammic acids, median (SE), mg/day | 66.3 (1.3) | 105.9 (0.9) | 154.4 (1.1) | 243.0 (4.8) |
| Model 1, OR (95% CI) a | 1 | 1.00 (0.57–1.77) | 0.35 (1.78–0.71) | 0.43 (0.20–0.92) |
| Model 2, OR (95% CI) b | 1 | 0.83 (0.43–1.61) | 0.26 (0.11–0.59) | 0.35 (0.14–0.88) |
| Model 3, OR (95% CI) c | 1 | 0.83 (0.43–1.62) | 0.26 (0.11–0.62) | 0.35 (0.13–0.91) |
| Hydroxyphenylacetic acids, median (SE), mg/day | 0.0 (0.0) | 0.2 (0.0) | 0.4 (0.004) | 0.8 (0.1) |
| Model 1, OR (95% CI) a | 1 | 0.93 (0.51–1.68) | 0.42 (0.20–0.88) | 0.82 (0.43–1.58) |
| Model 2, OR (95% CI) b | 1 | 0.47 (0.23–0.97) | 0.26 (0.11–0.61) | 0.45 (0.18–1.10) |
| Model 3, OR (95% CI) c | 1 | 0.48 (0.24–0.99) | 0.26 (0.11–0.63) | 0.46 (0.19–1.11) |
| Caffeic acid, median (SE), mg/day | 0.4 (0.0) | 0.8 (0.0) | 1.4 (0.0) | 3.3 (0.1) |
| Model 1, OR (95% CI) a | 1 | 0.51 (0.28–0.93) | 0.26 (0.13–0.53) | 0.46 (0.24–0.89) |
| Model 2, OR (95% CI) b | 1 | 0.57 (0.27–1.18) | 0.28 (0.12–0.63) | 0.31 (0.11–0.92) |
| Model 3, OR (95% CI) c | 1 | 0.58 (0.28–1.21) | 0.29 (0.12–0.68) | 0.32 (0.11–0.93) |
| Cinnamic acid, median (SE), mg/day | 0.0 (0.0) | 0.1 (0.0) | 0.2 (0.0) | 0.5 (0.1) |
| Model 1, OR (95% CI) a | 1 | 0.82 (0.43–1.54) | 0.98 (0.52–1.84) | 0.74 (0.38–1.44) |
| Model 2, OR (95% CI) b | 1 | 0.63 (0.29–1.36) | 1.02 (0.50–2.08) | 0.57 (0.27–1.22) |
| Model 3, OR (95% CI) c | 1 | 0.65 (0.30–1.39) | 1.04 (0.51–2.11) | 0.60 (0.28–1.29) |
| Vanillic acid, median (SE), mg/day | 0.1 (0.0) | 0.1 (0.0) | 0.4 (0.0) | 0.8 (0.0) |
| Model 1, OR (95% CI) a | 1 | 0.50 (0.26–0.96) | 0.51 (0.27–0.96) | 0.59 (0.31–1.14) |
| Model 2, OR (95% CI) b | 1 | 0.34 (0.15–0.74) | 0.46 (0.21–1.00) | 0.43 (0.17–1.05) |
| Model 3, OR (95% CI) c | 1 | 0.34 (0.15–0.74) | 0.48 (0.22–1.06) | 0.45 (0.18–1.11) |
| Ferulic acid, median (SE), mg/day | 0.6 (0.0) | 1.4 (0.0) | 2.7 (0.0) | 5.5 (0.2) |
| Model 1, OR (95% CI) a | 1 | 0.65 (0.35–1.20) | 0.75 (0.42–1.35) | 0.32 (0.14–0.73) |
| Model 2, OR (95% CI) b | 1 | 0.73 (0.36–1.47) | 1.04 (0.50–2.15 | 0.39 (015–0.98) |
| Model 3, OR (95% CI) c | 1 | 0.76 (0.37–1.55) | 1.13 (0.53–2.39) | 0.42 (0.16–1.07) |
a Model 1 adjusted for energy intake (kcal/day, continuous); b Model 2 = Model 1 + adjusted for BMI (continuous), smoking status (smokers, ex-smokers, non-smokers), alcohol consumption (0 g/day, <12 g/day, ≥12 g/day), physical activity level (low, medium, high), educational level (low, medium, high); c Model 3 = Model 2 + adherence to the Mediterranean diet. OR (odds ratio); CI (confidence interval).
The association between major dietary sources of phenolic acids and cognitive status is shown in Table 3. Individuals in the highest category of consumption of coffee (OR = 0.44 (95% CI: 0.20, 0.98)) and tea (OR = 0.30 (95% CI: 0.12, 0.72)) were less likely to have impaired cognitive status. On the contrary, no significant association was found for olive oil, nuts and beer intake.
Table 3.
Association between major dietary food sources of phenolic acids and impaired cognitive status.
| Food Group Intake, OR (95% CI) a | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Coffee b | 1 | 0.52 (0.12–2.14) | 0.96 (0.45–2.04) | 0.44 (0.20–0.98) |
| Nuts c | 1 | 1.52 (0.71–3.27) | 1.19 (0.54–2.62) | 1.16 (0.48–2.77) |
| Tea d | 1 | 0.76 (0.38–1.53) | 0.39 (0.18–0.82) | 0.30 (0.12–0.72) |
| Olive oil e | 1 | 1.01 (0.43–2.35) | 1.37 (0.60–3.08) | - |
| Beer f | 1 | 0.81 (0.42–1.57) | 1.22 (0.43–3.47) | 1.63 (0.43–6.15) |
a OR adjusted for sex, age (years, continuous), energy intake (kcal/day, continuous), smoking status (smokers, ex-smokers, non-smokers), alcohol consumption (0 g/day, <12 g/day, ≥12 g/day), physical activity level (low, medium, high), educational level (low, medium, high); b categories of coffee intake were as follows: Q1, less than 3 cups/week; Q2, 3 cups/week to 1 cup/day; Q3, more than 1 to less than 3 cups/day Q4, more than 3 cups/day; c categories of nuts intake were as follows: Q1, none; Q2, less than 1 serving (28 g)/day; Q3, 1–2 servings/day; Q4, more than 2 servings/day; d categories of tea intake were as follows: Q1, none; Q2, less than 1 cup/week; Q3, more than 1 cup/week to 1 cup/day; Q4, more than 1 cup/day; e categories of olive oil intake were as follows: Q1, less than 1 serving (1 spoon)/day; Q2, ½ to 1 serving/day; Q3, more than 1 serving/day; f categories of beer intake were as follows: Q1, none; Q2, less than 1 can/week; Q3, 1–3 cans/week; Q4, more than 3 cans/week.
4. Discussion
To our knowledge, this is the first observational study to examine the association between total dietary phenolic acids and their individual compounds intake and cognitive status. We observed that higher intakes of total phenolic acids and hydroxycinnamic acids were significantly inversely associated with cognitive impairment. Furthermore, among individual compounds, caffeic acid and ferulic acid showed a potential beneficial effect toward cognitive status.
Up to date, solely two observational studies investigated the association between dietary phenolic acid intake and cognitive status [34,35]. A prospective cohort study of 806 elderlies followed-up for 6 years showed a significant inverse association between higher dietary phenolic acid intake and cognitive decline, however, a clear linear trend in the association was not observed [34]. The results of SU.VI.MAX study conducted on 2574 middle-aged adults demonstrated that higher intake of hydroxybenzoic acids was positively associated with language and verbal memory, suggesting that diet high in phenolic acids may favor preservation of the verbal memory [35].
In accordance with the literature, the results of this study showed that intake of beverages containing phenolic acids, such as coffee and tea, may significantly impact cognitive status [36,37]. However, when interpreting these results it should be taken into consideration that the average daily intake of phenolic acids and their major food sources greatly differs across European populations [29,38,39]: in fact, to our knowledge, the present study is the first reporting such results in a population with very low consumption of tea, and relatively low consumption of coffee, compared to northern European countries, thus these results should be interpreted in this context.
Consistent with evidence from experimental studies, the results found in the present study suggest that individuals with high intake of hydroxycinnamic acids, as well as individual compounds such as caffeic acid and ferulic acid are less likely to have impaired cognitive status. From a mechanistic point of view, phenolic acids may affect cognitive function through various pathways including both direct and indirect modes of action. Phenolic acids have been shown to modulate different factors associated with the pathogenesis of neurodegenerative diseases, such as systemic inflammation and oxidative stress, and thus indirectly affect cognitive health [40]. As suggested by the evidence from experimental studies, specific phenolic acids and their metabolites (i.e., caffeic acid, ferulic acid) have been found in cerebrospinal fluid, suggesting that these compounds may cross the blood–brain barrier and thus directly affect brain cells [41,42]. Moreover, new evidence suggests that there is an interaction between dietary polyphenols and gut microbiota and their effect toward the brain [43,44]: it has been determined that gut microbiota composition significantly affects the bioavailability of polyphenols including phenolic acids and consequently may mediate and modulate the effect of dietary polyphenols toward cognitive health [45,46].
Concerning the direct effects of phenolic acids on the central nervous system, it has been demonstrated that hydroxycinnamic acids and their esters may exert beneficial effects toward cognitive functions by improving neuronal cell antioxidant activity against oxidative stress [47,48], through various signaling pathways including Nrf2/HO-1 pathway [49]. Moreover, hydroxycinnamic acids have been shown to rescue memory deficits in animal models of diabetes induced by streptozotocin through the reduction of oxidative stress and the positive modulation of the PI3-kinase pathway [50,51]. Among hydroxybenzoic acids, gallic acid has been shown to reverse impaired learning and memory in a rodent model of Alzheimer’s disease through simultaneous elevation of α-secretase and reduction of β-secretase activity and, most importantly, activation of metalloproteinase domain-containing protein 10 [52]. Furthermore, its role in modulation of neuroinflammation and the prevention of brain oxidative stress has been suggested. Recent studies also suggest that gallic acid can bind beta-amyloid monomers and prevent beta-amyloid aggregation, a primary event in the pathophysiology of Alzheimer’s disease [53]. Similarly, an increase in hippocampal dendritic spines and decreased oxidative stress and inflammation has been observed after administration of gallic acid in rats with metabolic syndrome [54], indicating that gallic acid could also play a protective role in the prevention of cognitive impairment triggered, at least partially, by metabolic disorders. Ellagic acid, which belongs to hydroxybenzoic acids, has been shown to exert neuroprotective effects by modulating neuroinflammation is a promising constituent for improving cognitive impairment [55] and alleviating memory impairment [56]. The neuroprotective activity of ellagic acid has been also validated in APP/PS1 mice, a validated transgenic animal model of Alzheimer’s disease, where it prevents neuronal apoptosis in the hippocampus and rescues memory deficits through the inhibition of beta-amyloid production and tau hyperphosphorylation [57].
Ferulic acid has been shown to promote neural differentiation and neurite outgrowth by exerting antiapoptotic and neuronal differentiation-inducing effects in a dose-dependent manner [58], while caffeic acid esters were found to influence the expression of brain-derived neurotrophic factor, a neurotrophic factor implicated in neuron survival, adult neurogenesis and synaptic plasticity [59]. The neuroprotective activity of ferulic acid is well-established and it is currently adopted as a potential pharmacophore in drug discovery processes for Alzheimer’s disease due to its multimodal mechanism of action which combines antioxidant and antiaggregant effects against Aβ oligomers with a Cholinesterase inhibition and a significant anti-inflammatory activity [60]. Different ferulic acid-based hybrid analogs are currently studied as multitarget directed ligands to develop novel hybrid compounds against Alzheimer’s disease [60].
Notwithstanding the novelty of this study, which for the first time explored the association between total phenolic acid and their individual components intake and cognitive status among the adult population, the results should be considered in light of some limitations.
First, given the cross-sectional design of the study, we cannot define the causal relation. Second, despite adjustment for potential confounders, including dietary factors, even though unlikely, residual confounding may be possible. However, after adjustment for the adherence to the Mediterranean diet most of the associations remained significant. Third, the use of FFQs may be susceptible to recall bias and potential inaccuracy in estimating dietary intake at least partially attributable to interpersonal variability in phenolic acids intake directly related to food quality (i.e., plant variety, season and environmental factors, food storage and processing). Finally, interindividual differences in the bioavailability and physiological response to phenolic acids exposure cannot be established [61].
5. Conclusions
In the present Mediterranean cohort, greater intakes of dietary phenolic acids were significantly inversely associated with impaired cognition. This association was confirmed for different phenolic acid subclasses and several individual compounds, emphasizing the importance of investigating a broad range of compounds in future studies to better elucidate their effect toward cognitive function. If confirmed, these findings may have important implications for cognitive decline prevention, given that the availability of modifiable risk factors which may impact cognition is limited.
Author Contributions
Conceptualization, J.G. and G.G.; methodology, J.G. and G.G.; formal analysis and investigation, J.G., A.M. and G.G.; writing—original draft preparation, J.G., F.C., A.M., S.C. and G.G.; writing—review and editing, J.G., F.C., A.M., S.C., E.D., N.P., R.F., F.G. and G.G.; visualization, A.M. and N.P.; supervision, F.C., R.F., F.G. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
The study was a part of the ADICOS (Association between DIetary factors and COgnitive Status) project funded by the “PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022—Starting Grant” of the University of Catania, Italy (J.G., G.G.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of CE Catania 2; (protocol code 802/23 December 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelino D., Godos J., Ghelfi F., Tieri M., Titta L., Lafranconi A., Marventano S., Alonzo E., Gambera A., Sciacca S., et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019;70:652–667. doi: 10.1080/09637486.2019.1571021. [DOI] [PubMed] [Google Scholar]
- 4.Tieri M., Ghelfi F., Vitale M., Vetrani C., Marventano S., Lafranconi A., Godos J., Titta L., Gambera A., Alonzo E., et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020;71:668–677. doi: 10.1080/09637486.2020.1715354. [DOI] [PubMed] [Google Scholar]
- 5.Martini D., Godos J., Marventano S., Tieri M., Ghelfi F., Titta L., Lafranconi A., Trigueiro H., Gambera A., Alonzo E., et al. Nut and legume consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2021:1–8. doi: 10.1080/09637486.2021.1880554. [DOI] [PubMed] [Google Scholar]
- 6.Rajaram S., Jones J., Lee G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019;10:S422–S436. doi: 10.1093/advances/nmz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosso G., Godos J., Galvano F., Giovannucci E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017;37:131–156. doi: 10.1146/annurev-nutr-071816-064941. [DOI] [PubMed] [Google Scholar]
- 8.Yi M., Wu X., Zhuang W., Xia L., Chen Y., Zhao R., Wan Q., Du L., Zhou Y. Tea Consumption and Health Outcomes: Umbrella Review of Meta-Analyses of Observational Studies in Humans. Mol. Nutr. Food Res. 2019;63:e1900389. doi: 10.1002/mnfr.201900389. [DOI] [PubMed] [Google Scholar]
- 9.Marino M., Del Bo C., Martini D., Porrini M., Riso P. A review of registered clinical trials on dietary (poly)phenols: Past efforts and possible future directions. Foods. 2020;9:1606. doi: 10.3390/foods9111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godos J., Vitale M., Micek A., Ray S., Martini D., Del Rio D., Riccardi G., Galvano F., Grosso G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants. 2019;8:152. doi: 10.3390/antiox8060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X.-F., Ruan Y., Li Z.-H., Li D. Flavonoid subclasses and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019;59:2850–2862. doi: 10.1080/10408398.2018.1476964. [DOI] [PubMed] [Google Scholar]
- 12.Grosso G., Micek A., Godos J., Pajak A., Sciacca S., Galvano F., Giovannucci E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017;185:1304–1316. doi: 10.1093/aje/kww207. [DOI] [PubMed] [Google Scholar]
- 13.Micek A., Godos J., Del Rio D., Galvano F., Grosso G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021;65:e2001019. doi: 10.1002/mnfr.202001019. [DOI] [PubMed] [Google Scholar]
- 14.Neveu V., Perez-Jiménez J., Vos F., Crespy V., du Chaffaut L., Mennen L., Knox C., Eisner R., Cruz J., Wishart D., et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database. 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godos J., Sinatra D., Blanco I., Mulè S., La Verde M., Marranzano M. Association between Dietary Phenolic Acids and Hypertension in a Mediterranean Cohort. Nutrients. 2017;9:1531. doi: 10.3390/nu9101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rienks J., Barbaresko J., Oluwagbemigun K., Schmid M., Nöthlings U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018;108:49–61. doi: 10.1093/ajcn/nqy083. [DOI] [PubMed] [Google Scholar]
- 17.Grosso G., Stepaniak U., Micek A., Stefler D., Bobak M., Pająk A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017;56:1409–1420. doi: 10.1007/s00394-016-1187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomone F., Ivancovsky-Wajcman D., Fliss-Isakov N., Webb M., Grosso G., Godos J., Galvano F., Shibolet O., Kariv R., Zelber-Sagi S. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep. 2020;2:100069. doi: 10.1016/j.jhepr.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godos J., Castellano S., Ray S., Grosso G., Galvano F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules. 2018;23:999. doi: 10.3390/molecules23050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godos J., Ferri R., Castellano S., Angelino D., Mena P., Del Rio D., Caraci F., Galvano F., Grosso G. Specific Dietary (Poly)phenols Are Associated with Sleep Quality in a Cohort of Italian Adults. Nutrients. 2020;12:1226. doi: 10.3390/nu12051226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosso G., Marventano S., D’Urso M., Mistretta A., Galvano F. The Mediterranean healthy eating, ageing, and lifestyle (MEAL) study: Rationale and study design. Int. J. Food Sci. Nutr. 2017;68:577–586. doi: 10.1080/09637486.2016.1262335. [DOI] [PubMed] [Google Scholar]
- 22.Mistretta A., Marventano S., Platania A., Godos J., Galvano F., Grosso G. Metabolic profile of the Mediterranean healthy Eating, Lifestyle and Aging (MEAL) study cohort. Med. J. Nutrition Metab. 2017;10:131–140. doi: 10.3233/MNM-17143. [DOI] [Google Scholar]
- 23.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Buscemi S., Rosafio G., Vasto S., Massenti F.M., Grosso G., Galvano F., Rini N., Barile A.M., Maniaci V., Cosentino L., et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int. J. Food Sci. Nutr. 2015;66:426–438. doi: 10.3109/09637486.2015.1025718. [DOI] [PubMed] [Google Scholar]
- 25.Marventano S., Mistretta A., Platania A., Galvano F., Grosso G. Reliability and relative validity of a food frequency questionnaire for Italian adults living in Sicily, Southern Italy. Int. J. Food Sci. Nutr. 2016;67:857–864. doi: 10.1080/09637486.2016.1198893. [DOI] [PubMed] [Google Scholar]
- 26.Castiglione D., Platania A., Conti A., Falla M., D’Urso M., Marranzano M. Dietary micronutrient and mineral intake in the mediterranean healthy eating, ageing, and lifestyle (MEAL) study. Antioxidants. 2018;7:79. doi: 10.3390/antiox7070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marventano S., Godos J., Platania A., Galvano F., Mistretta A., Grosso G. Mediterranean diet adherence in the Mediterranean healthy eating, aging and lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2018;69:100–107. doi: 10.1080/09637486.2017.1332170. [DOI] [PubMed] [Google Scholar]
- 28.Sofi F., Dinu M., Pagliai G., Marcucci R., Casini A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017;68:757–762. doi: 10.1080/09637486.2017.1287884. [DOI] [PubMed] [Google Scholar]
- 29.Godos J., Marventano S., Mistretta A., Galvano F., Grosso G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017;68:750–756. doi: 10.1080/09637486.2017.1285870. [DOI] [PubMed] [Google Scholar]
- 30.Godos J., Rapisarda G., Marventano S., Galvano F., Mistretta A., Grosso G. Association between polyphenol intake and adherence to the Mediterranean diet in Sicily, southern Italy. NFS J. 2017;8:1–7. doi: 10.1016/j.nfs.2017.06.001. [DOI] [Google Scholar]
- 31.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 32.Erkinjuntti T., Sulkava R., Wikström J., Autio L. Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J. Am. Geriatr. Soc. 1987;35:412–416. doi: 10.1111/j.1532-5415.1987.tb04662.x. [DOI] [PubMed] [Google Scholar]
- 33.Pilotto A., Ferrucci L. Verso una definizione clinica della fragilità: Utilità dell’approccio multidimensionale. G Gerontol. 2011;59:125–129. [Google Scholar]
- 34.Goni L., Fernández-Matarrubia M., Romanos-Nanclares A., Razquin C., Ruiz-Canela M., Martínez-González M.Á., Toledo E. Polyphenol intake and cognitive decline in the Seguimiento Universidad de Navarra (SUN) Project. Br. J. Nutr. 2020:1–24. doi: 10.1017/S000711452000392X. [DOI] [PubMed] [Google Scholar]
- 35.Kesse-Guyot E., Fezeu L., Andreeva V.A., Touvier M., Scalbert A., Hercberg S., Galan P. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J. Nutr. 2012;142:76–83. doi: 10.3945/jn.111.144428. [DOI] [PubMed] [Google Scholar]
- 36.Liu X., Du X., Han G., Gao W. Association between tea consumption and risk of cognitive disorders: A dose-response meta-analysis of observational studies. Oncotarget. 2017;8:43306–43321. doi: 10.18632/oncotarget.17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q.-P., Wu Y.-F., Cheng H.-Y., Xia T., Ding H., Wang H., Wang Z.-M., Xu Y. Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta-analysis of prospective cohort studies. Nutrition. 2016;32:628–636. doi: 10.1016/j.nut.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Grosso G., Stepaniak U., Topor-Mądry R., Szafraniec K., Pająk A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition. 2014;30:1398–1403. doi: 10.1016/j.nut.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamora-Ros R., Knaze V., Rothwell J.A., Hémon B., Moskal A., Overvad K., Tjønneland A., Kyrø C., Fagherazzi G., Boutron-Ruault M.-C., et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016;55:1359–1375. doi: 10.1007/s00394-015-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grabska-Kobylecka I., Kaczmarek-Bak J., Figlus M., Prymont-Przyminska A., Zwolinska A., Sarniak A., Wlodarczyk A., Glabinski A., Nowak D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence That Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients. 2020;12:1531. doi: 10.3390/nu12051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obrenovich M.E., Donskey C.J., Schiefer I.T., Bongiovanni R., Li L., Jaskiw G.E. Quantification of phenolic acid metabolites in humans by LC-MS: A structural and targeted metabolomics approach. Bioanalysis. 2018;10:1591–1608. doi: 10.4155/bio-2018-0140. [DOI] [PubMed] [Google Scholar]
- 43.Ceppa F., Mancini A., Tuohy K. Current evidence linking diet to gut microbiota and brain development and function. Int. J. Food Sci. Nutr. 2019;70:1–19. doi: 10.1080/09637486.2018.1462309. [DOI] [PubMed] [Google Scholar]
- 44.Salvucci E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019;70:781–795. doi: 10.1080/09637486.2019.1580682. [DOI] [PubMed] [Google Scholar]
- 45.Frolinger T., Sims S., Smith C., Wang J., Cheng H., Faith J., Ho L., Hao K., Pasinetti G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019;9:3546. doi: 10.1038/s41598-019-39994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mena P., Bresciani L. Dietary fibre modifies gut microbiota: what’s the role of (poly)phenols? Int. J. Food Sci. Nutr. 2020;71:783–784. doi: 10.1080/09637486.2020.1826913. [DOI] [PubMed] [Google Scholar]
- 47.Garrido J., Gaspar A., Garrido E.M., Miri R., Tavakkoli M., Pourali S., Saso L., Borges F., Firuzi O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie. 2012;94:961–967. doi: 10.1016/j.biochi.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Rehman S.U., Ali T., Alam S.I., Ullah R., Zeb A., Lee K.W., Rutten B.P.F., Kim M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019;56:2774–2790. doi: 10.1007/s12035-018-1280-9. [DOI] [PubMed] [Google Scholar]
- 49.Morroni F., Sita G., Graziosi A., Turrini E., Fimognari C., Tarozzi A., Hrelia P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in A Mouse Model of Alzheimer’s Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018;9:605–622. doi: 10.14336/AD.2017.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemmati A.A., Alboghobeish S., Ahangarpour A. Effects of cinnamic acid on memory deficits and brain oxidative stress in streptozotocin-induced diabetic mice. Korean J. Physiol. Pharmacol. 2018;22:257–267. doi: 10.4196/kjpp.2018.22.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar M., Bansal N. Caffeic acid phenethyl ester rescued streptozotocin-induced memory loss through PI3-kinase dependent pathway. Biomed. Pharmacother. 2018;101:162–173. doi: 10.1016/j.biopha.2018.02.089. [DOI] [PubMed] [Google Scholar]
- 52.Mori T., Koyama N., Yokoo T., Segawa T., Maeda M., Sawmiller D., Tan J., Town T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020;295:16251–16266. doi: 10.1074/jbc.RA119.012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nie R.-Z., Huo Y.-Q., Yu B., Liu C.-J., Zhou R., Bao H.-H., Tang S.-W. Molecular insights into the inhibitory mechanisms of gallate moiety on the Aβ1-40 amyloid aggregation: A molecular dynamics simulation study. Int. J. Biol. Macromol. 2020;156:40–50. doi: 10.1016/j.ijbiomac.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Diaz A., Muñoz-Arenas G., Caporal-Hernandez K., Vázquez-Roque R., Lopez-Lopez G., Kozina A., Espinosa B., Flores G., Treviño S., Guevara J. Gallic acid improves recognition memory and decreases oxidative-inflammatory damage in the rat hippocampus with metabolic syndrome. Synapse. 2020;75:e22186. doi: 10.1002/syn.22186. [DOI] [PubMed] [Google Scholar]
- 55.Dornelles G.L., de Oliveira J.S., de Almeida E.J.R., Mello C.B.E., Rodrigues B.R.E., da Silva C.B., Petry L.D.S., Pillat M.M., Palma T.V., de Andrade C.M. Ellagic acid inhibits neuroinflammation and cognitive impairment induced by lipopolysaccharides. Neurochem. Res. 2020;45:2456–2473. doi: 10.1007/s11064-020-03105-z. [DOI] [PubMed] [Google Scholar]
- 56.Wang W., Yang L., Liu T., Wang J., Wen A., Ding Y. Ellagic acid protects mice against sleep deprivation-induced memory impairment and anxiety by inhibiting TLR4 and activating Nrf2. Aging. 2020;12:10457–10472. doi: 10.18632/aging.103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong L., Liu H., Zhang W., Liu X., Jiang B., Fei H., Sun Z. Ellagic acid ameliorates learning and memory impairment in APP/PS1 transgenic mice via inhibition of β-amyloid production and tau hyperphosphorylation. Exp. Ther. Med. 2018;16:4951–4958. doi: 10.3892/etm.2018.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moghadam F.H., Mesbah-Ardakani M., Nasr-Esfahani M.H. Ferulic Acid exerts concentration-dependent anti-apoptotic and neuronal differentiation-inducing effects in PC12 and mouse neural stem cells. Eur. J. Pharmacol. 2018;841:104–112. doi: 10.1016/j.ejphar.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Kurauchi Y., Hisatsune A., Isohama Y., Mishima S., Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012;166:1151–1168. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh Y.P., Rai H., Singh G., Singh G.K., Mishra S., Kumar S., Srikrishna S., Modi G. A review on ferulic acid and analogs based scaffolds for the management of Alzheimer’s disease. Eur. J. Med. Chem. 2021;215:113278. doi: 10.1016/j.ejmech.2021.113278. [DOI] [PubMed] [Google Scholar]
- 61.Mena P., Del Rio D. Gold standards for realistic (poly)phenol research. J. Agric. Food Chem. 2018;66:8221–8223. doi: 10.1021/acs.jafc.8b03249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.