Inverse hydroboration of pyridinesa.
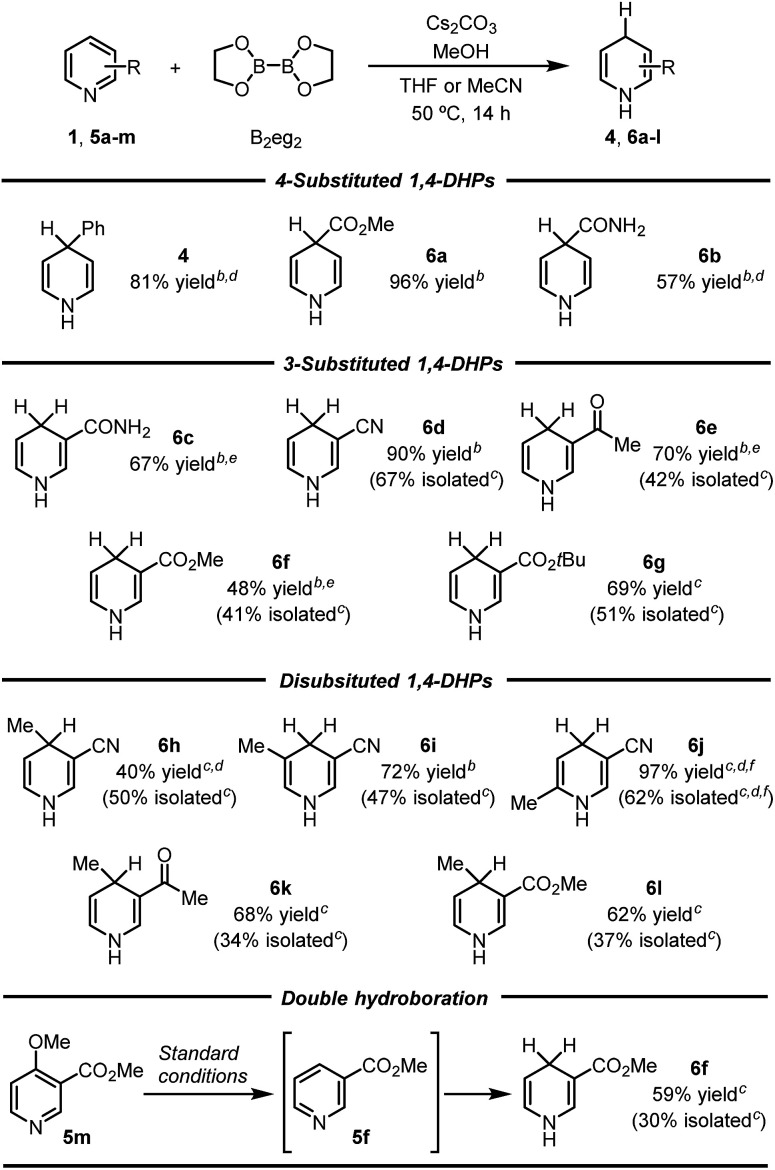
|
Reaction conditions: pyridine substrate (1 equiv.), B2eg2 (1.1 equiv.), Cs2CO3 (1.1 equiv.), MeOH (11 equiv.), sealed tube, 50 °C for 14 h. Yields of the 1,4-DHP products were determined by 1H NMR analysis of the reaction mixture using DMSO as the internal standard (isolated yields are shown in parentheses).
THF as the solvent.
MeCN as the solvent.
MeONa was used instead of Cs2CO3.
MeOK and 18-crown-6 (1.1 equiv. each) were used instead of Cs2CO3.
The reaction was conducted at 65 °C.
