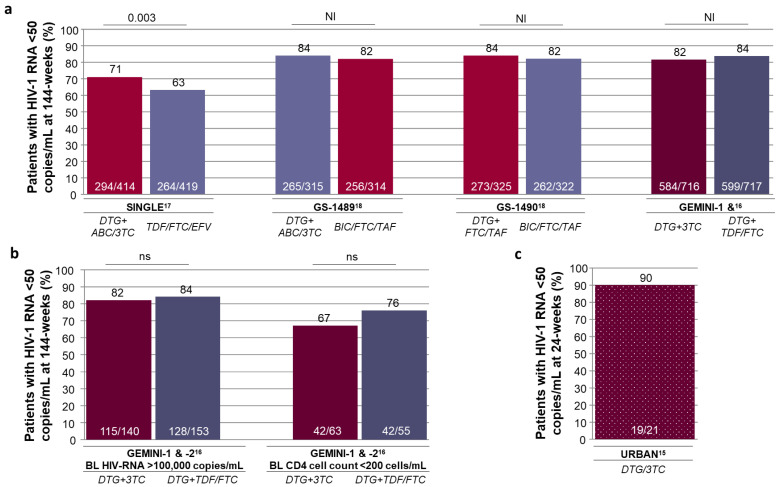
Figure 1.
Dolutegravir plus lamivudine two- and three-drug regimens in treatment-naïve patients. (a) Efficacy in randomized clinical trials by FDA snapshot analysis at week-144. Histograms represent individual studies—not a head-to-head comparison. (b) Efficacy of dolutegravir plus lamivudine in GEMINI-1 and GEMINI-2 randomized clinical trials by FDA snapshot analysis at week-144, in patients with baseline viral load >100,000 copies/mL, and in patients with baseline CD4 cell count <200 copies/mL. (c) Efficacy of dolutegravir plus lamivudine in URBAN real-life study, week-24 result in the effectiveness set (excluding missing data). 3TC = lamivudine; ABC = abacavir; BIC = bictegravir; BL = baseline; DTG = dolutegravir; EFV = efavirenz; FTC = emtricitabine; NI = noninferior treatment difference; ns = not statistically significant difference; TAF = tenofovir alafenamide; TDF = tenofovir disoproxil fumarate.