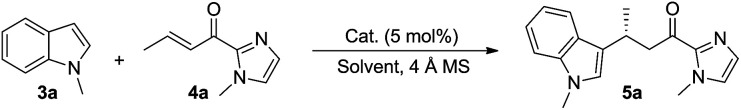
Screening of the catalysts for the asymmetric alkylation of N-methylindole 3a with α,β-unsaturated 2-acyl imidazole 4aa.
| |||||
|---|---|---|---|---|---|
| Entry | Cat. | Solvent/T (°C) | T (h) | % Yb | % eec |
| 1 | 1a | CH2Cl2/25 | 3 | 90 | 65 |
| 2d | 1a | CH2Cl2/25 | 4 | 92 | 60 |
| 3e | 1a | CH2Cl2/25 | 5 | 88 | 58 |
| 4 | 1a | CH2Cl2/5 | 10 | 91 | 73 |
| 5 | 1b | CH2Cl2/5 | 10 | 98 | 79 |
| 6 | 1c | CH2Cl2/5 | 15 | 90 | 69 |
| 7 | 1d | CH2Cl2/5 | 12 | 87 | 57 |
| 8 | 1e | CH2Cl2/5 | 10 | 99 | 85 |
| 9 | 1f | CH2Cl2/5 | 10 | 94 | 71 |
| 10 | 1e | CH2Cl2/−15 | 24 | 95 | 90 |
| 11 | 1e | ClCH2CH2Cl/−15 | 24 | 97 | 93 |
| 12 | 1e | CH3Cl/−15 | 24 | 96 | 91 |
| 13 | 1e | CH3CN/−15 | 20 | 92 | 95 |
| 14 | 1e | CH3CN/−25 | 48 | 91 | 97.5 |
Reaction conditions: 3a (0.45 mmol), 4a (0.3 mmol), 1 (5 mol%), 4 Å MS (100 mg) and solvent (3 mL), under argon.
Isolated yield.
Determined by chiral-phase HPLC.
Without 4 Å MS.
Without 4 Å MS, under air.
