Abstract
Coronavirus disease 2019 (COVID-19) is associated with irreversible effects on vital organs, especially the respiratory and cardiac systems. While the immune system plays a key role in the survival of patients to viral infections, in COVID-19, there is a hyperinflammatory immune response evoked by all the immune cells, such as neutrophils, monocytes, and includes release of various cytokines, resulting in an exaggerated immune response, named cytokine storm. This severe, dysregulated immune response causes multi-organ damage, which eventually leads to high mortality. One of the most important components of hypersensitivity is immunoglobulin E (IgE), which plays a major role in susceptibility to respiratory infections and can lead to the activation of mast cells. There is also a negative association between IgE and IFN-α, which can reduce Toll-like receptor (TLR) nine receptor expression and TLR-7 signaling to disrupt IFN production. Moreover, anti-IgE drugs such as omalizumab reduces the severity and duration of COVID-19. In addition to its anti-IgE effect, omalizumab inhibits inflammatory cells such as neutrophils. Hence, blockade of IgE may have clinical utility as an immunotherapy for COVID-19.
Keywords: COVID-19, IgE, immunity system, hyperinflammation, hypersensitivity, omalizumab
Introduction
Coronavirus disease 2019 (COVID-19), as a deadly pandemic, has killed more than 1.5 million people so far [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has irreversible effects on most of the organs, especially the cardiorespiratory system, even in people who have recovered [2,3]. Widespread prevalence of the virus has led to major restrictions for people to prevent its progression [4,5]. These restrictions have imposed severe economic and cultural damage to human societies [6,7]. Therefore, this disease has endangered not only physical, but also mental health [8,9]. One of the challenges of this disease is a severe drop in blood oxygen levels, and many efforts have been made to improve it with the help of drugs and ventilators. This hypoxia is profound and disproportionate and causes vascular dysfunction in the later stages of the disease. This requires an additional vascular and rheological approach, which in turn makes the use of anticoagulants in the treatment of COVID-19 a necessity [10]. Additional complexity of this disease is its numerous clinical manifestations, in which 11 different faces of the disease have been observed so far: patients' symptoms can be categorized in a wide range from asymptomatic from mild to severe, with or without pneumonia [11]. Despite many efforts, the vaccine or definitive cures for the disease has been challenged so far. Therefore, only supportive therapies are available for COVID-19 patients and the main role in patients' survival is still on responsibility for their immune system [12–15]. Therefore, most of the studies have examined the role of the host immune system and its management against the influenza virus and COVID-19 [16–19].
Hypersensitivity of the immune system and COVID-19
In COVID-19, the immune system acts like a double edge sword, because on the one hand, effective immunity is required to fight the virus, and on the other hand, severe inflammation leads to multi-organ damage and is one of the major causes of patient mortality [20,21]. COVID-19 seems to have a three-stage progression. The first stage is the initial infection, the second stage is the pulmonary phase and the third stage is the inflammatory phase. The initial stage is attributed to the virus (5–7 days), while the next two stages are thought to be due to the inflammatory response (7–15 days from the onset of the disease) [22]. In fact, SARS-CoV-2 infection differs in several epidemiological and pathological features from many other viral infections. One of them is the high level of cytokine release, which in turn causes an uncontrollable reaction known as the ‘cytokine storm’. This phenomenon contributes to Acute Respiratory Distress Syndrome (ARDS), leading to pneumonia and respiratory failure. After the virus reaches the lung tissue, innate immunity drives the inflammatory cascade that fight against pathogens; in this case, SARS-CoV-2. However, this inflammatory activation also leads to severe cardiorespiratory system damage [23]. Therefore, fighting hyper-inflammation and regulating immune function play a very important role in controlling the pathogenesis of COVID-19. Hyper-inflammation in response to viral infection seems to be the main cause of its lethality [24,25]. For this reason, the mechanism of sensitization by viral infections of the respiratory tract is an important research area [26]. In this regard, because of their anti-inflammatory effects, corticosteroids have become an adjunctive therapy for acute respiratory syndrome and help to effectively treat the phenomenon of cytokine storm in COVID-19 [27]. One of the reasons for using this group of drugs is the successful experience of their use in the face of coronavirus 1 (respiratory syndrome) coronavirus (SARS-CoV-1) [28]. Also, clinical observational studies have shown improvement in symptoms and oxygenation in patients with severe COVID-19 who have been treated with corticosteroids: the mortality rate in the corticosteroid group was significantly lower than the non-corticosteroid group (4.3% vs. 15.6%) [29].
Hypersensitivity, asthma, IgE, and infection
One of the most important components of immune hypersensitivity, is IgE, which is also involved in allergies, which comprise the hyper-reactivity of the immune system to a variety of factors. People with allergies have immune systems that overreact to seemingly harmless substances in their habitat. IgE activity releases heparin, histamine, and some other cytokines. These substances also cause systemic reactions, especially in the respiratory tract, causing asthma, which is somewhat similar to the respiratory distress observed in COVID-19 [30]. Therefore, considering the essential role of IgE in allergies, and their relationship with viral infections, it is crucial to study its role in COVID-19 infection [31]. Indeed, in vitro studies have shown that the production of specific IgE for different viruses and the ability of IgE to suppress some viruses indicate an important role of IgE/or specific IgE expression against the virus in viral pathogenesis [32]. One of the most important classes of allergy is asthma, and the risk of developing asthma increases with recurrent respiratory infections [33]. For example, the development of asthma after respiratory syncytial virus (RSV) bronchiolitis has been reported in case–control studies. However, the determinants of asthma after RSV and its mechanisms are not clearly known yet [34]. The effect of RSV-specific IgE antibodies in the pathophysiology of virus-induced airway dysfunction has been assessed in a mouse model. The results of this study suggest that RSV-specific IgE may contribute to the pathophysiology of airway dysfunction in children who develop this class of specific antibodies [35]. Moreover, data from recent mouse models suggest that the development of asthma following a severe respiratory viral infection may be arise through the production of antiviral IgE. Hence, this new paradigm helps to find and developing future therapies to prevent or improving atopic disease after the virus [36]. Thus, IgE might represent a new marker for human viral diseases, and IgE may also play a functional role in the pathogenesis of the viral diseases [37,38].
IgE, and COVID-19 in asthma patients
Acute exacerbation of asthma (as in patients with high IgE) cause increased morbidity and health care costs. Since respiratory viral infections can exacerbate asthma [39,40], the symptoms of SARS-CoV-2 infection may be more severe in patients with asthma. However, existing studies have not shown the expected prevalence in asthma patients among patients with COVID-19 [41]. Some aspects of the type-2 immune response, including type-2 cytokines (IL-4, IL-13, etc.) and eosinophil accumulation, may provide potential protective effects against COVID-19 [42,43]. In particular, it has been reported that blood eosinopenia is observed in more than half of acute COVID-19 patients, both in severe and less severe cases. Normalization of eosinophil counts showed improvement in clinical status in a number of other cases [44]. Eosinopenia has been shown to be inversely related to inflammatory markers and can be associated with the severity of COVID-19 [45]. For this reason, in many clinical studies, asthma has not yet been identified as a risk factor for severe outcomes in COVID-19 [46]. On the other hand, asthma and influenza are separate diseases in terms of immunity. Examination of B cell responses in mice with asthma and infected with influenza virus showed that allergic response and airway inflammation were the predominant responses. However, virus-specific IgE antibodies have also been induced by the B cells that mentioned above [47]. So far, it seems that higher levels of IgE are an advantage for protecting against SARS-CoV-2. However, it should be noted that apparent protective effects related to higher expression of IgE in asthmatic patients is the result of the higher activity of their humoral immune system rather than higher levels of IgE. Because, increasing in IgE expression can leads to exacerbation of various syndromes [48]. On the other hand, the flu vaccine also causes IgE sensitivity in young children, which may make them more susceptible to vaccine-induced anaphylaxis. This was rarely seen after vaccination in the H1N1 flu epidemic. This can impose a major challenge for vaccine design and use for this disease [49,50]. In addition, incomplete production of IFNs by pDC and epithelial cells have been observed in severely atopic patients with delayed and ineffective antiviral defenses. Also, IFNs act as negative regulators of Th2 evolution, differentiation, and function. There is also a negative association between IgE and IFN-α, because IgE cross-linking reduces Toll-like receptor (TLR) 9 expression and TLR-7 signaling, that inhibit the production of type I and III INF from pDCs [51]. One of the attractive goals that can completely change the prognosis of COVID-19 patients is disrupting the activation process of mast cells. Numerous studies provide documentary evidence of the importance of mast cell involvement in the pathogenesis and progression of COVID-19. This occurs by completing the inflammatory process by creating a proper niche of anti-inflammatory cytokines in the infected tissue. Mast cell activation also occurs with the help of IgE; so, it seems that IgE blockade helps to treat patients with COVID-19 [52].
COVID-19, omalizumab and other anti IgE drugs
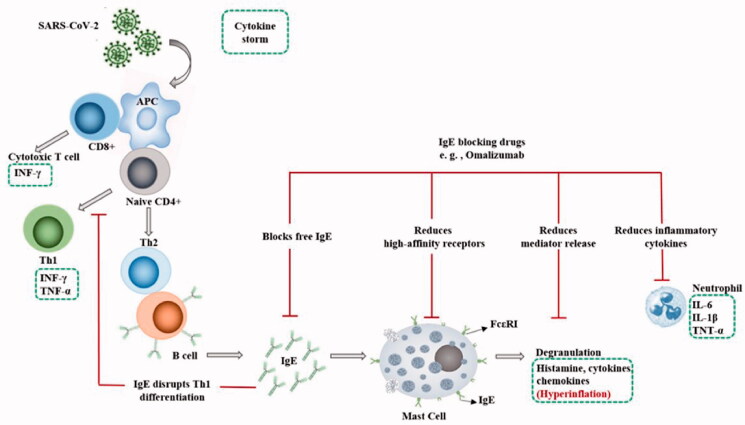
Omalizumab is a recombinant human anti-IgE antibody originally designed to reduce sensitivity to allergens, and blocks IgE binding to the high-affinity IgE receptor (FcεRI). It also inhibits FcεRI -associated with activation in mast cells by removing surface IgE. Omalizumab prevents IgE and IgE/anti-IgE-dependent degranulation, as well as receptor expression which is done by human mast cells. Additionally, omalizumab can even detach IgE pre-bound to sensitized mast cells. As a result, their response to FcεRI-dependent signals is reduced. These data suggest that omalizumab is an effective inhibitor of sensitized and insensitive mast cells [53] (Figure 1). Defects in cellular antiviral responses is one of the mechanisms that link viral infections to exacerbations of atopic disease. For example, the stimulation of the FcεRI has been shown to inhibit influenza-induced IFN-α secretion. Hence, it has been reported that a decrease in serum IgE by using omalizumab in allergic patients with asthma can reverse the ex vivo antiviral responses to IFN-α and improve clinical outcomes. Allergic stimulation through IgE-dependent binding affects the functions of monocyte apoptosis, cytokine secretion, and phagocytosis. Thus, defining the effects of IgE on monocyte antiviral responses could reveal pathways involved in the pathogenesis of atopic disease. The proposed mechanism is that allergic stimulation significantly disrupts the rearrangement of proteins essential for antigen delivery. As a result, stimulation of T lymphocytes is weaker and TH1 antiviral responses are reduced. Also, considering, the association of IgE effects that inhibit virus-induced TH1 can be expressed. Thus, IgE-regulated degradation of antiviral responses in human monocytes might suppress the maturation of virus-induced monocytes and TH1 differentiation (Figure 1). All mentioned above highlights our knowledge about variation of allergen-related pathways in host antiviral responses and indicates the role of monocytes in exacerbations of virus-associated allergic disease [54]. Meanwhile, therapeutic role of IgE blocking drugs in RSV viral infection has already been well established [55,56]. For example, in a clinical trial in children with allergic asthma, treatment with omalizumab reduced the duration of RSV infection, viral shedding, and reduced the risk of developing RSV. These findings provide direct evidence that the IgE blockade reduces the susceptibility to RSV infection and disease [57]. While IgE is involved at the beginning of the inflammatory cascade and can be supposed of as the cause of allergic asthma, eosinophilia can be considered a consequence of the whole process [58]. A case study of a 52-year-old man during COVID-19 with severe allergic asthma treated with omalizumab was also reported, which no evidence of exacerbation of asthma or loss of asthma control or pneumonia was reported. Therefore, IgE-targeting antibodies may have protective effects against viruses in COVID-19 [59]. While corticosteroids reduce in-hospital mortality [60], the drugs are highly immunosuppressive, increasing the risk of bacterial superinfection and resistance to neuromuscular blocking agents, which are widely used in mechanical ventilation in patients with SARS [28]. As a result, an attempt has been made to use corticosteroids by the inhalation method to apply it, and reduce the using dose to prevent its side effects [61]. Thus, IgE blockade might be able to prevent the adverse effects of corticosteroids, which target the entire immune system, and might better balance corticosteroid-induced immunosuppression such that the immune system's response to the virus or other infections remains intact. In fact, corticosteroids have a function similar to omalizumab. Prednisolone at a dose of 1 mg/kg can reduce IgE in patients with asthma with or without pulmonary aspergillosis [62]. However, IgE induces heparin secretion, which is one of the common anticoagulants to neutralize the coagulation effect of Coronavirus. Therefore, the use of IgE blocking drugs should be used with caution, and heparin or another anticoagulant should be adjusted with it [63]. IgE blockers can be utilized as a type of immunotherapy along with conventional drugs in order to cure COVID-19 patients, although this treatment still under investigation [64]. The positive effects of biologic drugs have been proven well for patients with severe asthma. Even in patients who have not been cured with other therapies, biological dugs have shown significant results. Currently, available biological drugs are available as adjunctive therapy for patients with severe asthma included Omalizumab, Mepolizumab, Benralizumab, Reslizumab, and Dupilumab. These biological drugs target type-2 inflammatory pathways and are effective in reducing exacerbation, controlling asthma symptoms, and leading to reduce the use of systemic steroids [65]. Among these, Omalizumab is better known. Because not only it has been shown to reduce inflammation of the nasal mucosa and improve nasal breathing, but it has also been shown to improve nasal sinus function in patients with chronic rhinosinusitis. This mechanism is essential for the initial fight against COVID-19. Also, it can be used to treat patients with a variety of mast cell disorders, even in small doses. Moreover, its usage reduces the spread of the virus due to proinflammatory mediators and the risk of subsequent serious complications in COVID-19 patients. Furthermore, the drug not only has an anti-IgE effect, but also has an inhibitory effect on inflammatory cells such as neutrophils. Also, considering that the over-synthesis of inflammatory cytokines such as IL-6, IL-1β and tumor necrosis factor (TNF)-α are caused by active neutrophils. It can be concluded that Anti-IgE monoclonal antibodies based drugs can have a significant effect in inhibiting further inflammation and preventing cytokine storm [66] (Figure 1). Also, analyzing preliminary data in a clinical study demonstrated that tocilizumab significantly improved the clinical outcome in severe and critical COVID-19 patients immediately, and it is an effective treatment to reduce mortality in COVID-19 [67].
Figure 1.
The role of IgE in SARS-CoV-2 induced cytokine storm and IgE blocking drugs (e.g. omalizumab) during immune response.
Conclusion
One of the leading causes of death from COVID-19 is inflammation and severe allergies caused by the virus. Among them, IgE is one of the most important components of sensitivity. Also, production of specific IgE for different viruses and its important role in post-infection allergies by activating mast cells, as well as its function in inhibiting TNF-α activity dysregulated immune response showing an important role IgE is involved in the exacerbation of viral diseases. Therefore, the use of IgE suppressors can improve the symptoms and reduce the severity of the disease in patients. Therefore, it can be concluded that more attention to IgE and further research on its role in COVID-19 infection and other similar viral diseases can create new horizons in the treatment of viral infections.
Acknowledgments
The authors are very appreciating from Professor Stephen J. Polyak, Professor in Departments of Global Health and Microbiology, in University of Washington, for his comments and critical edition of this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Hanaei S, Rezaei N.. COVID-19: Developing from an outbreak to a pandemic. Arch Med Res. 2020;51(6):582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renu K, Prasanna PL, Valsala Gopalakrishnan A.. Coronaviruses pathogenesis, comorbidities and multi-organ damage – A review. Life Sci. 2020;255:117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong T-Y, Redwood S, Prendergast B, et al. . Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan Sun M, Lan Cheong Wah CB.. Lessons to be learnt from the COVID-19 public health response in Mauritius. Public Health Pract. 2020;1:100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vally Z. Public perceptions, anxiety, and the perceived efficacy of health-protective behaviours to mitigate the spread of the SARS-Cov-2/COVID-19 pandemic. Public Health. 2020;187:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva PCL, Batista PVC, Lima HS, et al. . COVID-ABS: An agent-based model of COVID-19 epidemic to simulate health and economic effects of social distancing interventions. Chaos, Solitons Fractals. 2020;139:110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan Y, Deng H, Zhou X.. Understanding the impact of the COVID-19 pandemic on career development: Insights from cultural psychology. J Vocat Behav. 2020;119:103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur V, Jain A.. COVID 2019-suicides: A global psychological pandemic. Brain Behav Immun. 2020;88:952–953. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Elbay RY, Kurtulmuş A, Arpacıoğlu S, et al. . Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 2020;290:113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendjelid K, Giraud R, Von Düring S.. Treating hypoxemic COVID-19 “ARDS” patients with almitrine: The earlier the better? Anaesthesia, Crit Care Pain Med. 2020;39(4):451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong X, Cao Y, Lu X, et al. . Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momtazmanesh S, Ochs HD, Uddin LQ, et al. . All together to Fight COVID-19. The Am J Trop Med Hygiene 2020;102(6):1181–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saghazadeh A, Rezaei N.. Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti-antibodies, immunoglobulins, and corticosteroids. Int Immunopharmacol. 2020;84:106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shereen MA, Khan S, Kazmi A, et al. . COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saghazadeh A, Rezaei N.. Immune-epidemiological parameters of the novel coronavirus–a perspective. Expert Rev Clin Immunol. 2020;16(5):465–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang T, Bidon M, Jaimes JA, et al. . Coronavirus membrane fusion mechanism offers as a potential target for antiviral development. Antiviral Res. 2020; 178:104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip T-F, Selim ASM, Lian I, et al. . Advancements in host-based interventions for influenza treatment. Front Immunol. 2018;9:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catanzaro M, Fagiani F, Racchi M, et al. . Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig Transduct Target Ther. 2020;5(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury MA, Hossain N, Kashem MA, et al. . Immune response in COVID-19: A review. J Infect Public Health. 2020;13(11):1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazdanpanah F, Hamblin MR, Rezaei N.. The immune system and COVID-19: Friend or foe? Life Sci. 2020;256:117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azkur AK, Akdis M, Azkur D, et al. . Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taboada M, Caruezo V, Naveira A, et al. . Corticosteroids and the hyper-inflammatory phase of the COVID-19 disease. J Clin Anesth. 2020;66:109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdin SM, Elgendy SM, Alyammahi SK, et al. . Tackling the cytokine storm in COVID-19, challenges, and hopes. Life Sci. 2020; 257:118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song YG, Shin H-S.. COVID-19, a clinical syndrome manifesting as hypersensitivity pneumonitis. Infect Chemother. 2020;52(1):110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roncati L, Ligabue G, Fabbiani L, et al. . Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol. 2020; 217:108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martorano LM, Grayson MH.. Respiratory viral infections and atopic development: From possible mechanisms to advances in treatment. Eur J Immunol. 2018;48(3):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamloo F, König R.. SARS‐CoV‐2 immunogenicity at the crossroads. Allergy. 2020;75(7):1822–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattos-Silva P, Felix NS, Silva PL, et al. . Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J-W, Yang L, Luo R-G, et al. . Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. 2020;26(9):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbas AK, Lichtman AH, Pillai S.. Cellular and molecular immunology E-book. Elsevier Health Sci. 2014:417–434. [Google Scholar]
- 31.Klimek L, Jutel M, Akdis C, et al. . Handling of allergen immunotherapy in the COVID‐19 pandemic: An ARIA‐EAACI statement. Allergy. 2020;75(7):1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith-Norowitz TA, Wong D, Kusonruksa M, et al. . Long term persistence of IgE anti-influenza virus antibodies in pediatric and adult serum post vaccination with influenza virus vaccine. Int J Med Sci. 2011;8(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards MR, Strong K, Cameron A, et al. . Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140(4):909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacharier LB, Cohen R, Schweiger T, et al. . Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130(1):91–100. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dakhama A, Park J-W, Taube C, et al. . The role of virus-specific immunoglobulin E in airway hyperresponsiveness. Am J Respir Crit Care Med. 2004;170(9):952–959. [DOI] [PubMed] [Google Scholar]
- 36.Khan SH, Park SS, Sirajuddin IA, et al. . Respiratory virus and asthma: the role of immunoglobulin E. Clin Ther. 2008;30:1017–1024. [DOI] [PubMed] [Google Scholar]
- 37.Smith-Norowitz TA, Kusonruksa M, Wong D, et al. . Long-term persistence of IgE anti-influenza A HIN1 virus antibodies in serum of children and adults following influenza A vaccination with subsequent H1N1 infection: a case study. J Inflammat Res. 2012;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacofsky D, Jacofsky EM, Jacofsky M.. Understanding antibody testing for covid-19. J Arthroplasty. 2020;35(7):S74–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bønnelykke K, Vissing NH, Sevelsted A, et al. . Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136(1):81–86. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver BG, Robinson P, Peters M, et al. . Viral infections and asthma: an inflammatory interface? Eur Respiratory Soc. 2014;44(6):1666–1681. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J-j, Dong X, Cao Y-y, et al. . Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan. China. Allergy. 2020;75(7):1730–1741. [DOI] [PubMed] [Google Scholar]
- 42.Avdeev S, Moiseev S, Brovko M, et al. . Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19. Allergy. 2020;75(10):2703–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Zhi Y, Ying S.. COVID-19 and asthma: reflection during the pandemic. Clinic Rev Allerg Immunol. 2020;59(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jesenak M, Banovcin P, Diamant Z.. COVID‐19, chronic inflammatory respiratory diseases and eosinophils–Observationsfrom reported clinical case series. Allergy. 2020;75(7):1819–1822. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L, Zhang Y, Yang X, et al. . Eosinopenia is associated with greater severity in patients with coronavirus disease 2019. Allergy. 2021;76(2):562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston SL. Asthma and COVID‐19: is asthma a risk factor for severe outcomes? Allergy. 2020;75(7):1543–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doorley LA, LeMessurier KS, Iverson AR, et al. . Humoral immune responses during asthma and influenza co-morbidity in mice. Immunobiology. 2017;222(12):1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergerson JR, Freeman AF.. An update on syndromes with a hyper-IgE phenotype. Immunol Allergy Clin. 2019;39(1):49–61. [DOI] [PubMed] [Google Scholar]
- 49.Rouleau I, De Serres G, Drolet JP, et al. . Allergic symptoms after pandemic influenza vaccination rarely mediated by vaccine-specific IgE. J Allergy Clin Immunol. 2012;130(6):1423–1426. [DOI] [PubMed] [Google Scholar]
- 50.Nagao M, Fujisawa T.. Inactivated influenza vaccine induces IgE antibody to the vaccine in preschool children. J Allergy Clin Immunol. 2019;143(2):AB228. [Google Scholar]
- 51.Carli G, Cecchi L, Stebbing J, et al. . Is asthma protective against COVID‐19. Allergy. 2021;76(3):866–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theoharides TC. COVID‐19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors (Oxford, England). 2020;46(3):306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakami T, Blank U.. From IgE to omalizumab. JI. 2016;197(11):4187–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowe RK, Pyle DM, Tomlinson AR, et al. . IgE cross-linking impairs monocyte antiviral responses and inhibits influenza-driven TH1 differentiation. J Allergy Clin Immunol. 2017;140(1):294–298. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jartti T, Bønnelykke K, Elenius V, et al. . Role of viruses in asthma. Semin Immunopathol. 2020;42(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jartti T, Smits HH, Bønnelykke K, EAACI Task Force on Clinical Practice Recommendations on Preschool Wheeze, et al.. Bronchiolitis needs a revisit: distinguishing between virus entities and their treatments. Allergy. 2019;74(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esquivel A, Busse WW, Calatroni A, et al. . Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196(8):985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matucci A, Vultaggio A, Maggi E, et al. . Is IgE or eosinophils the key player in allergic asthma pathogenesis? Respir Res. 2018;19(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lommatzsch M, Stoll P, Virchow JC.. COVID‐19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75(10):2705–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bani-Sadr F, Hentzien M, Pascard M, et al. . Corticosteroid therapy for patients with CoVID-19 pneumonia: a before-after study. Int J Antimicrob Agents. 2020;56(2):106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bousquet J, Akdis C, Jutel M, the ARIA‐MASK Study Group, et al.. Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: An ARIA‐EAACI statement. Allergy. 2020;75(10):2440–2444. [DOI] [PubMed] [Google Scholar]
- 62.Lin RY, Joshi S.. Pulse corticosteroids as an omalizumab enabling IgE reduction modality in an asthmatic without allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2015;135(2):AB2. [Google Scholar]
- 63.Gelincik A, Brockow K, Çelik GE, et al. . Diagnosis and management of the drug hypersensitivity reactions in Coronavirus disease 19. Allergy. 2020;75(11):2775–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pashaei M, Rezaei N.. Immunotherapy for SARS-CoV-2: Potential Opportunities. Taylor and Francis. 2020;20(10):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morais-Almeida M, Aguiar R, Martin B, et al. . COVID-19, asthma, and biologic therapies: What we need to know. World Allergy Organ J. 2020;13(5):100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdelmaksoud A, Goldust M, Vestita M.. Omalizumab and COVID19 Treatment: Could It Help? Dermatologic Ther. 2020;33(4):e13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu X, Han M, Li T, et al. . Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]