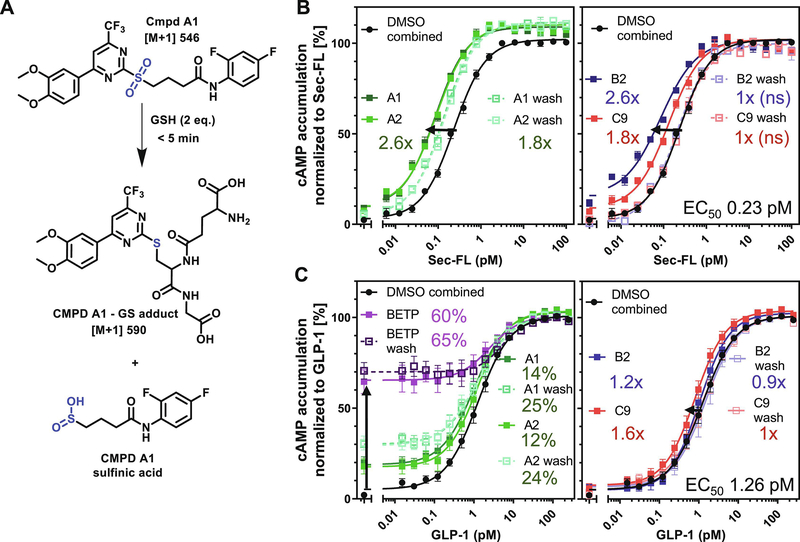
Figure 7: Scaffold A but not B and C demonstrate electrophilic reactivity and potential covalent mechanism of action:
(A) Covalent reaction of glutathione (GSH) and A1 leading to the formation CMPD A1- GS adduct and CMPD A1 sulfinic acid confirmed by HPLC-MS analysis. cAMP dose-response curves of (B) Sec-FL stimulating SCTRs and (C) GLP-1 stimulating GLP-1Rs; both pre-treated (dotted lines) prior a triple wash step with DMSO, irreversible scaffold A (A1 wash, A2 wash, BETP wash, left panels) or reversible scaffolds (B2 wash, C9 wash, right panels) as well as co-treated (solid lines) with DMSO, irreversible scaffold A (A1, A2, BETP, left panels) or reversible scaffolds (B2, C9, right panels) added after wash; TR-FRET ratios resulting from cAMP accumulation normalized to corresponding agonist; graphs plotted using GraphPad Prism; experiments performed in duplicate in at least three independent experiments; data points shown as mean ± SEM. Statistical significance determined using GraphPad Prism’s unpaired t-test corrected with Holm-Sidak method, α = 0.01; (B) all potency shifts statistically significant (p < 0.01) if not indicated otherwise (ns = not significant); (C) EC50 shifts for all conditions not statistically significant except BETP wash (p < 0.01) and C9 (p < 0.05). Intrinsic activity (% basal activity) statistically significant (p < 0.01) for BETP, BETP wash, A1, A1 wash, A2 and A2 wash.