Abstract
Genetic analysis of brown midrib sorghum (Sorghum bicolor) mutant lines assembled in our program has previously shown that the mutations fall into four allelic groups, bmr2, bmr6, bmr12 or bmr19. Causal genes for allelic groups bmr2, bmr6 and bmr12, have since been identified. In this report, we provide evidence for the nature of the bmr19 mutation. This was accomplished by introgressing each of the four bmr alleles into nine different genetic backgrounds. Polymorphisms from four resequenced bulks of sorghum introgression lines containing either mutation, relative to those of a resequenced bulk of the nine normal midrib recurrent parent lines, were used to locate their respective causal mutations. The analysis confirmed the previously reported causal mutations for bmr2 and bmr6 but failed in the case of bmr12-bulk due to a mixture of mutant alleles at the locus among members of that mutant bulk. In the bmr19-bulk, a common G → A mutation was found among all members in Sobic.001G535500. This gene encodes a putative folylpolyglutamate synthase with high homology to maize Bm4. The brown midrib phenotype co-segregated with this point mutation in two separate F2 populations. Furthermore, an additional variant allele at this locus obtained from a TILLING population also showed a brown midrib phenotype, confirming this locus as Bmr19.
Keywords: sorghum, brown midrib mutants, bmr19, lignin, lignocellulosic feedstock, folylpolyglutamate synthase
1. Introduction
1.1. Biological Role of Lignin
Lignin is a complex heteropolymer of phenylpropanoid-derived monolignols providing support and water-conducting ability to the cell wall by cross-linking with its polysaccharide components cellulose (polymers of glucose) and hemicellulose (heteropolymers of other monosaccharides including arabinose, glucose, mannose and xylose). Together these cell wall components form the lignocellulosic matrix that constitutes the greatest part of dry plant matter. The lignocellulosic matrix varies greatly between plant species and organs of individual plants [1]. The cross linking provided by lignin in grass secondary cell walls allows herbaceous panicoids to reach up to several meters in height. Leaf lignin content is highest in sheaths and midribs of the leaf blades making these more rigid to support a plant architecture that maximizes photosynthetic capacity [2,3]. The lignin constituent is particularly diverse, involving several possible linkages among the basic monolignol subunits [1].
1.2. Significance of Lignin in Biofuel Production
Among the many ethnobotanical uses of lignocellulosic biomass is feedstock in the production of ethanol as an environmentally friendly alternative to liquid fossil fuels. Carbon emissions of lignocellulosic ethanol is arguably half of gasoline when considered over the entire production and use chain [4]. Both technical and transport logistic concerns limit its economic viability, however. Chief among these concerns is the processing needed to expose the cellulose and hemicellulose to saccharification, thereby releasing their sugars for fermentation to ethanol. Lignin not only shields the polysaccharides from hydrolysis, it also binds the added hydrolytic enzymes, which impedes saccharification [5]. Feedstock from tissues of highly lignified cell walls require more input for the same ethanol yield than less fortified tissues.
1.3. Biosynthesis of Lignin, Composition of Monolignols and Their Polymerization
Monolignol subunits differ in the number of methyoxy groups on their aromatic rings, syringyl (S) having two, guaiacyl (G) with one and, peculiar to grasses, p-hydroxyphenyl (H) subunits with none [5]. The cinnamyl alcohol dehydrogenase (CAD2) catalyzes the reduction of hydroxycinnamyl aldehydes from their corresponding alcohols (monolignols) [6]. The aldehyde precursors are synthesized in the cytosol from phenylalanine or tyrosine through a series of steps, one of which is catalyzed by 4-coumarate: coenzyme A ligase (4CL) [7]. Two O-methyl transferases, caffeoyl coenzyme A 3-O-methyl transferase (CCoAOMT) and caffeic acid-O-methyl transferase (COMT) catalyze the addition of methoxy groups at 3 and 5 positions of phenol ring using S-adenosyl methionine (SAM) as the methyl donor [8]. Polymerization of monolignols occurs within the cell wall through radical-coupled oxidation of the monolignols [9]. In grasses, phenolic compounds p-coumaric acid and ferulic acid derived from this pathway are also incorporated into cell walls and can be linked to lignin polymers [2].
1.4. Brown Midrib Mutations
Mutations affecting lignin biosynthesis in maize (Zea mays), sorghum (Sorghum bicolor) and pearl millet (Pennisetum glaucum) phenotypes are identified by the reddish-brown leaf midribs and stalk fibers [10]. Recently, this phenotype was also reported in rice (Oryza sativa) carrying a mutation in a CAD2 gene [11]. The cause of this color change from the normal green or white is not understood, but this change may be due to incorporation of hydroxycinnamaldehydes into the lignin polymer [12].
1.5. Allelic Groups among the bmr Mutants of Sorghum
An original set of 19 sorghum brown midrib (bmr) mutants were first described in our sorghum research program from two lines chemically mutagenized in efforts to improve forage quality [13]. Since then, we have added a few more lines through mutagenesis and selections from natural variants in different source populations. A total of 28 brown midrib sorghum variants were later subjected to an allelism test determining that the collection of mutants we had assembled fall into four allelic groups (bmr2, bmr6, bmr12 and bmr19) with altered lignin content [14]. An additional four allelic groups (bmr29 through bmr32) have also been isolated subsequently [15].
The Bmr2 gene of sorghum was identified as Sobic.004G062500, encoding a 4CL and two distinct missense mutations in Bmr2 were described [7]. Leaf midrib lignin content of bmr2 mutants is 16% less than wildtype [14]. Lignin of bmr2 mutants have about half the concentration of G-lignin as wildtype with a slight, though less consistent, reduction in S-lignin [14]. The glucose yield after saccharification with cellulose hydrolyzing enzymes was increased in bmr2 mutant biomass over wildtype by 17% [14].
The Bmr6 gene of sorghum was identified as Sobic.004G071000, encoding CAD2. Its homolog in maize is Bm1 [16]. At least eight distinct bmr6 alleles have been described [3,6,17,18,19]. Leaf midrib lignin content of bmr6 mutants is 19% less than wildtype with the G-lignin subunits disproportionately reduced relative to S-lignin [14]. Saccharification yields an average of 16% more glucose than wildtype in bmr6 mutants [14]. This translates to an increased ethanol conversion efficiency of 44% [20].
The Bmr12 gene of sorghum was identified as Sobic.007G047300, encoding a COMT [21] and its homolog in maize is Bm3 [22]. Nine distinct mutant bmr12 alleles have been identified [14,21,23,24,25]. Leaf midrib lignin content of bmr12 mutants averages about 13% less than wildtype [14]. The cell wall of the bmr12 mutants contains fewer S-lignin subunits and less esterified p-coumaric acid (preferentially esterified to S-lignin) relative to wildtype [14,21]. This translates to an increased ethanol conversion efficiency of 46% [20]. These mutants also have significantly less tricin than wildtype, a feature indicative of the hypothesized role of COMT in tricin biosynthesis [26]. Saccharification is improved by as much as 24% over wildtype in bmr12 mutants [14].
The Bmr19 gene of sorghum was not previously identified and only one allele was identified in allelism tests [14]. Leaf midrib lignin content of bmr19 mutants is only slightly (−3%) less than wildtype [14]. The bmr19 mutant midrib has a similar lignin composition to bmr2 mutants, having a more modest reduction in G-lignin and a slight reduction in S-lignin relative to wildtype [14]. Saccharification was not improved in bmr19 stover and may have even yielded less glucose than wildtype [14]. In this report, we identify the gene that encodes Bmr19 as a putative folylpolyglutamate synthase (FPGS) gene, which has high amino acid sequence similarity to maize Bm4 (Phytozome v.12). This evidence is based on whole-genome sequenced bulks from five different genetic backgrounds into which the four allelic groups were introgressed and their respective unconverted wild type parents (Table 1), as well as an additional bmr19 allele at this locus from an EMS mutagenized TILLING population with the brown midrib phenotype.
Table 1.
Constitutions of the resequenced bulks and pedigrees of brown midrib introgression lines.
| Normal (Non-Brown) Bulk | bmr2 Bulk | bmr6 Bulk | bmr12 Bulk | bmr19 Bulk | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pedigree | Relevant Alleles in Homozygous State | Pedigree | Relevant Alleles | Pedigree | Relevant Alleles | Pedigree | Relevant Alleles | Pedigree | Relevant Alleles |
| PP290 | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × PP290)BC3F5 | bmr2-ref | (bmr6ms3 × PP290)BC3F5 | bmr6-ref | (bmr18ms3 × PP290)BC3F5 | bmr12-18 | (bmr19ms3 × PP290)BC2F5 | bmr19-ref |
| PRL983999 | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × PRL983999)BC3F4 | bmr2-ref | (bmr6ms3 × PRL983999)BC3F4 | bmr6-ref | (bmr12ms3 × PRL983999)F4 | bmr12-ref | (bmr19ms3 × PRL983999)F4 | bmr19-ref |
| Sudan Zera-Zera | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × Sudan Zera-Zera)F4 | bmr2-ref | (bmr6ms3 × Sudan Zera-Zera)BC3F4 | bmr6-ref | (bmr18ms3 × Sudan Zera-Zera)BC3F4 | bmr12-18 | (bmr19ms3 × Sudan Zera-Zera)BC2F5 | bmr19-ref |
| PU216B | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × PU216B)BC2F5 | bmr2-ref | (bmr6ms3 × PU216B)BC3F5 | bmr6-ref | (bmr12ms3 × PU216B)BC3F5 | bmr12-ref | (bmr19ms3 × PU216B)BC3F3 | bmr19-ref |
| P942242B | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × P942242B)BC3F4 | bmr2-ref | (bmr6ms3 × P942242B)BC3F4 | bmr6-ref | (bmr18ms3 × P942242B)BC3F4 | bmr12-18 | (bmr19ms3 × P942242B)BC2F4 | bmr19-ref |
| P90344 | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr6ms3 × P90344)BC2F5 | bmr6-ref | (bmr18ms3 × P90344)BC2F5 | bmr12-18 | (bmr19ms3 × P90344)BC3F3 | bmr19-ref | ||
| P90812 | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × P90812)F4 | bmr2-ref | (bmr3ms3 × P90812)BC2F5 | bmr6-3 | (bmr12ms3 × P90812)BC2F5 | bmr12-ref | (bmr19ms3 × P90812)BC2F5 | bmr19-ref |
| Tx623B | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr2ms3 × Tx623B)BC3F5 | bmr2-ref | (bmr6ms3 × Tx623B)BC3F5 | bmr6-ref | (BTx623ms3 × bmr18)BC2F4 | bmr12-18 | (BTx623ms3 × bmr19)BC2F4 | bmr19-ref |
| P9401 | Bmr2, Bmr6, Bmr12, Bmr19 | (bmr5ms3 × P9401)BC4F5 | bmr2-5 | (bmr3ms3 × P9401)BC4F5 | bmr6-3 | (bmr7ms3 × P9401)BC4F5 | bmr12-7 | (P9401ms3 × bmr19)BC3F5 | bmr19-ref |
2. Materials and Methods
2.1. Plant Material
A group of introgression lines in which mutations in each of the original sorghum Bmr genes (bmr2-ref, bmr2-5, bmr6-ref, bmr6-3, bmr12-ref, bmr12-7, bmr12-18 and bmr19-ref) were backcrossed into nine different genetic backgrounds. The various brown midrib mutations were introduced in the original crosses using a genic male sterile (ms3ms3) version of each mutant donor as the female, except in a few crosses involving male sterile (ms3ms3) Tx623 and P9401 where the brown midrib line was used as the male (Table 1). From the F2 of each cross, selection was for the brown midrib phenotype and against male sterility. The donors of the bmr2 mutations mainly carried the bmr2-ref allele, except in one lineage in which bmr2-5 was used. The donors of bmr6 lineages included either of two alleles, bmr6-ref or bmr6-3. The donors of bmr12 lineages included either of three alleles, bmr12-ref, bmr12-7 or bmr12-18. The bmr19 donors all carried the original mutant allele (bmr19-ref). The male parents were chosen based on desirable agronomic characters and were pure lines with normal (non-brown) midrib phenotypes. These were used as recurrent parents during the backcrossing process.
2.2. DNA Extraction and Construction of the Bulked Pools
Two leaf discs of 0.6 cm diameter were punched from one plant representing each of the lineages, confirming the leaf midrib phenotype (brown or normal). Two leaf discs of nine plants (one from each lineage) were ground together according to the target Bmr gene in Table 1 with a mortar and pestle (16–18 discs) in liquid nitrogen. Five bulks were thus homogenized: the bmr2 bulk with plants with the bmr2/bmr2 genotype, the bmr6 bulk sharing the genotype bmr6/bmr6, the bmr12 bulk with bmr12/bmr12, the bmr19 bulk with bmr19/bmr19 and the normal bulk sharing wildtype alleles at all four Bmr loci (Table 1). DNA was isolated in 4 × 100 mg aliquots from each of the five homogenates in separate extractions using the Qiagen DNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA). The smaller aliquots avoided overloading the capacity of the extraction kit units while providing enough DNA for sequencing. Equimolar concentrations of DNA from each of the four aliquots representing each bulk were combined and purified with the DNA Clean-Up & Concentration Kit (ZYMO Research, Irvine, CA, USA) before submitting for sequencing at the Purdue Genomics Core Facility, West Lafayette, Indiana.
2.3. Construction of Libraries and Illumina Sequencing
Libraries were prepared using TruSeq DNA PCR-Free LT Library Preparation Kit-Set B, FC-121-3002 (Illumina, San Diego, CA, USA). Two micrograms of DNA from each sample was sheared using a Covaris S2 ultrasonicator (Covaris, Woburn, MA, USA), end repaired and adapter ligated. Each sample was ligated to differently indexed adapters to allow them all to be run in the sample lane. Size selection of libraries was performed using polyethylene glycol cuts with the aid of magnetic binding beads as described in the kit protocol. This results in a target insert size of 500–600 bp among library molecules that cluster. The quality of the resulting DNA libraries was assessed on an Agilent Technologies 2100 Bioanalyzer using a High Sensitivity chip. Final libraries were titred for clustering using a KAPA Library Quantification Kit Illumina® Platforms (KAPA Cat # KR045), pooled and clustered in 3.5 lanes of Illumina Hiseq 2500 High Output v3 chemistry (Illumina Inc., San Diego, CA, USA) to generate 100-base pair-end reads.
2.4. Construction of Reference-Based Assemblies
The statistics of generated sequencing reads was estimated using Samtools [27]. The Mutmap pipeline (http://genome-e.ibrc.or.jp/home/bioinformaticsteam/mutmap, accessed on 12 October 2019, developed by Iwate Biotechnology Research Center, Kitakami, Japan) was used for calculating the single nucleotide polymorphism (SNP)-index. Briefly, the cleaned reads of one the normal midrib parents which had been resequenced (Sudan Zera-Zera) were first aligned to the sorghum reference genome of BTx623 (PhytozomeV10: Sbicolor_313_v3.1.1) using the Burrows–Wheeler Aligner [28]. Coval 1.4 was used for post-processing and filtering of the alignment files [29]. The variants called were then used to develop a reference-based assembly of Sudan Zera-Zera by substituting the bases with confidence variants calls in the genome. The reads from the each of the bmr mutant bulks and the normal bulk were aligned separately and variants were called for both bulks against the developed reference assembly.
Calculation of SNP-index: a SNP-index for each SNP position was calculated for each bulk as per Abe et al. [30] using the formula:
| SNP-index (at a position) = count of alternate base/count of reads aligned. |
The SNPs in the candidate region close to the causal mutation are expected to have a higher SNP index (SNP-index ≈ 1); in contrast, those in unlinked regions show a SNP index of c. 0.5. A peak in the SNP index indicates the approximate genomic interval harboring the causal mutation. The positions with SNP-index < 0.3 and read depth < 0.7 in the bulks was filtered out for ΔSNP-index calculation. ΔSNP-index was calculated by subtracting SNP-index of each bmr bulk from SNP-index of the normal bulk. The ΔSNP-index is ≈1 if the bulked DNA comprises only bmr genome, ΔSNP-index is ≈ −1 if it is the normal parent genome only and ΔSNP-index = 0 if both bulks have the same SNP indices at the genomic regions. The possible effects of the SNPs (synonymous, missense and frameshift mutations) between the bmr19 and normal bulks at candidate genomic regions were inferred using the predictCoding tool in the GenomicFeatures [31] and VariantAnnotation [32] open source software in R. The selected variants were compared to the variant data of 71 sorghum lines [33,34] available on Phytozome V 12.1. Our expectation was that our candidate causal SNP would not be present in the variant data since none of the deep sequenced lines have a brown midrib phenotype. Using this approach, we were able to eliminate several variants in the candidate region until we found the causal variant for each mutation. Individual sequence reads containing the causal variants were also viewed with IGV software (v.2.8.10) [35] where closely linked SNPs were expected to show 100% mutant and 0% normal-type reads.
2.5. Indel Markers of the Bmr12 Gene
Variation among members of the bmr12 and normal bulks was examined by PCR followed by gel electrophoresis of the amplicons using primers that amplified two functionally inert indels within Sobic.007G047300 [18,36]. The largest indel, present in the first intron is an Olo24 retrotransposon present in the reference genome but absent in both of the lines (P954104 and P954114) mutagenized by Porter et al. [13] and therefore, also absent in the original 19 brown midrib mutant lines. This region was amplified with the primer pair (5′ → 3′) GACCGGACAGTGACTTCAGAG (forward) and GGACTGTTACTGCTGCCATGGC (reverse). Presence and absence of this element were distinguished by amplicons differing by 348 bp. The other polymorphism was in a SSR (TATC)n, also in the first intron, amplified by the primers TCCGAAGTGCTCAAGCCTAT (forward) and CAGTCGTGGAGGATCCACTT (reverse). The original brown midrib mutant lines vary from the reference at this position by two fewer repeat units (8 bp).
2.6. Validation of Identified bmr19 Mutant SNP
One genic SNP identified for the bmr19 mutation was validated in nine parents used in the normal bulk and the nine bmr19 lineages used to constitute the bmr19 bulk (Table 1). The SNP was also validated in two F2 populations, (bmr19 × IS4225)F2 (n = 285) and (BTx623 × bmr19)F2 (n = 149) segregating for the brown midrib phenotype. For this purpose, the tetra-primer ARMS-PCR technique [37] was used to design allele specific primers that converted the SNP to a size polymorphism that could be detected by gel electrophoresis. This marker was also used on the original brown midrib lines of our collection (bmr2, bmr3, bmr4, bmr6, bmr7, bmr12, bmr14, bmr15, bmr18, bmr19, bmr20, bmr22, bmr23, bmr24, bmr25, bmr26, bmr27 and bmr28) to check for co-segregation of the SNP with the bmr19-specific brown-midrib phenotype.
2.7. Alternate bmr19 Alleles
Because only one mutant allele, bmr19-ref, in this group was available in our stocks, we took advantage of the sequenced M4 lines of a TILLING population resulting from ethyl methanesulfonate (EMS) mutagenesis of BTx623. According to the Functional Gene Discovery Platform for Sorghum created at Purdue University (https://www.purdue.edu/sorghumgenomics/, accessed on 12 October 2019), six EMS lines carry point mutations in coding regions of Sobic.001G535500. Four of these were obtained from the U.S. National Plant Germplasm System (NPGS), accessions PI 677943 (0623), PI 677955 (0662), PI 678119 (2354) and PI 678132 (2465). These were grown in pots in the greenhouse along with bmr19 and BTx623 as mutant and wildtype checks, respectively. By six weeks after sowing, midrib phenotypes were visually scored.
The only one of these EMS lines showing a brown midrib phenotype, 2354, carrying an allele that we named bmr19-2, was crossed onto an emasculated bmr19 plant and the resulting seed was sown in greenhouse pots. The putative F1 plants were confirmed as non-selfs by checking their DNA with the tetra-primers [37] used to track segregation of the bmr19-ref and Bmr19 alleles in the F2 populations described in the validation section. True F1s give two allele specific amplicons (162 + 124 bp) by PCR, along with the control PCR product (233 bp), just like heterozygotes in the screened F2s. Progeny of any possible self-pollinated plants, therefore, would give two bands when their PCR products are separated on a gel: control (233 bp) and the bmr19-ref allele “A” at this position (162 bp), while the progeny of a cross gives three bands: control (233 bp), the bmr19-ref allele (162 bp) and the one for “G” at this position (124 bp). Because of the proximity of the bmr19-2 and bmr19-ref point mutations (Table 2), the control amplicon spanned the location of both point mutations as does the 124 bp product unique to F1 plants. Sequencing of this latter PCR product was used to confirm the presence of the bmr19-2 allele in the F1 plants. At six weeks, the midrib phenotype of these plants was visually scored.
Table 2.
Lines derived from ethyl methanesulfonate mutagenesis with variation in Sobic.001G535500 with respect to the reference genome.
| Line Name | NPGS Accession Number | SRA Accession Number | Mutation Position on Chr01 * | Base Pair Change | Amino Acid Change Chemical Characteristic Change |
Leaf Midrib Phenotype | Mutant Allele Name |
|---|---|---|---|---|---|---|---|
| 0623 | PI 677943 | SRR2759749 | 79,894,180 | C → T | A221V hydrophobic → hydrophobic |
normal | |
| 2354 | PI 678119 | SRR2759203 | 79,894,457 | G → A | E236K negative → positive |
brown | bmr19-2 |
| 2465 | PI 678132 | SRR2759494 | 79,895,561 | G → A | S378N polar → polar |
normal | |
| 0662 | PI 677955 | SRR2759755 | 79,894,460 | G → A | V237I nonpolar → nonpolar |
normal | |
| bmr19 | 79,894,470 | G → A | G240E nonpolar → negative |
brown | bmr19-ref |
* position according to Phytozome Sorghum bicolor v.3.1.1.
2.8. Biomass Composition Analysis
Two sets of plants were used to compare effects of bmr mutations on lignin amount in dried biomass after removing mature grain (stover). The first set, grown in the greenhouse in the off-season included all lines of the BTx623 background [BTx623, (bmr2ms3 × Tx623B)BC3F5, (bmr6ms3 × Tx623B)BC3F5, (BTx623ms3 × bmr18)BC2F4, (BTx623ms3 × bmr19)BC2F4 and BTx623 EMS line 2354]. The second set included only those lines contrasting for variations in Bmr19 [BTx623, (BTx623ms3 × bmr19)BC2F4 and BTx623 EMS line 2354]. This set was grown in the field during the regular season in West Lafayette. Above-ground plant parts were harvested at grain maturity, the panicles removed, stalks split length-wise and vegetative tissues (leaves and stems) chopped into 2” pieces and dried in a convection oven at 50⁰C for two weeks. Fiber analysis was performed on ground stover from each set to determine cell wall components using a detergent digestion protocol as described by Vogel et al. [38]. Neutral detergent fiber, acid detergent fiber and acid detergent lignin concentrations were estimated using the ANKOM 200 fiber analyzer (ANKOM Tech Co., Macedon, NY, USA) [38]. Relative percentage of individual cell wall components (cellulose, hemicellulose and lignin) were calculated using component concentrations extracted on a dry weight basis [39]. Stover from four biological replicates was analyzed in duplicate (technical reps) for the first set and five biological replicates in duplicate for the second set. Each biological replicate consisted of one plant. Stover from bmr19 and wildtype plants of the second set were treated for thioacidolysis followed by gas chromatography-mass spectrometry (GC-MS) to determine relative lignin subunit composition (p-hydroxyphenyl, guaiacyl and syringyl lignin). Samples were prepared and analyzed as described by Palmer et al. [40]. Analysis was performed in duplicate on five biological replicates per line. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis of compositional data. The linear mixed model analysis of variance (ANOVA) with the Tukey–Kramer multiple comparison adjustment procedure was performed to compare lignin properties between genotypes.
3. Results
3.1. Confirmation of the bmr2-ref Allele in the bmr2 Bulk
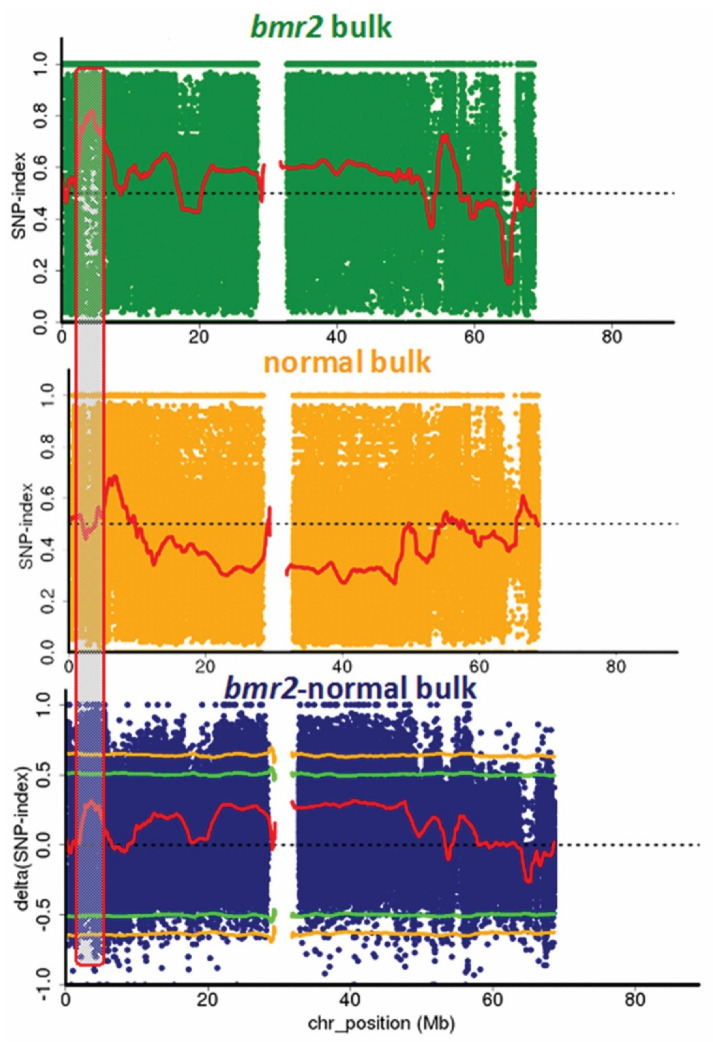
Mutmap applied to the bmr2 bulk revealed a SNP index peak in the genomic interval from 3 Mb to 6 Mb on Chromosome 4. Analysis of SNPs in the candidate region revealed the same SNP in Sobic.004G062500, encoding 4CL, identified by Saballos et al. [7] differentiating bmr2 from the normal bulk. Plots of the SNP indices of the bulks and ΔSNP-index between them are shown in Figure 1. The consensus assemblies of the two bulks show a SNP at position Chr04:5,041,758 (Phytozome Sorghum bicolor v.3.1.1) with G → A on the forward strand predicted to cause the missense mutation G111D in the 4CL protein, the bmr2-ref allele [7]. Most reads in the bmr2 bulk showed this allele (A) at this position while all those of the normal bulk had the wildtype allele (G) here. Donor lines of the brown midrib phenotype carried the bmr2-ref allele (Table 1), but one donor in this group was bmr5, a previous undefined mutant, which contains a C → T mutation at Chr04:5,042,645 resulting in the substitution H251Y called bmr2-5 [14].
Figure 1.
Single nucleotide polymorphism (SNP) analysis to identify bmr2 mutation. SNP index plots of bmr2 bulk (top), normal bulk (middle) and Δ(SNP-index) plot (bottom) of sorghum Chromosome 4 with statistical confidence intervals under the null hypothesis of no QTLs (green, p < 0.05; yellow, p < 0.01). Grey highlighted area shows the QTL near the beginning of Chromosome 4 where the significant difference between the bulks occurs.
3.2. Confirmation of the bmr6-ref Allele in the bmr6 Bulk
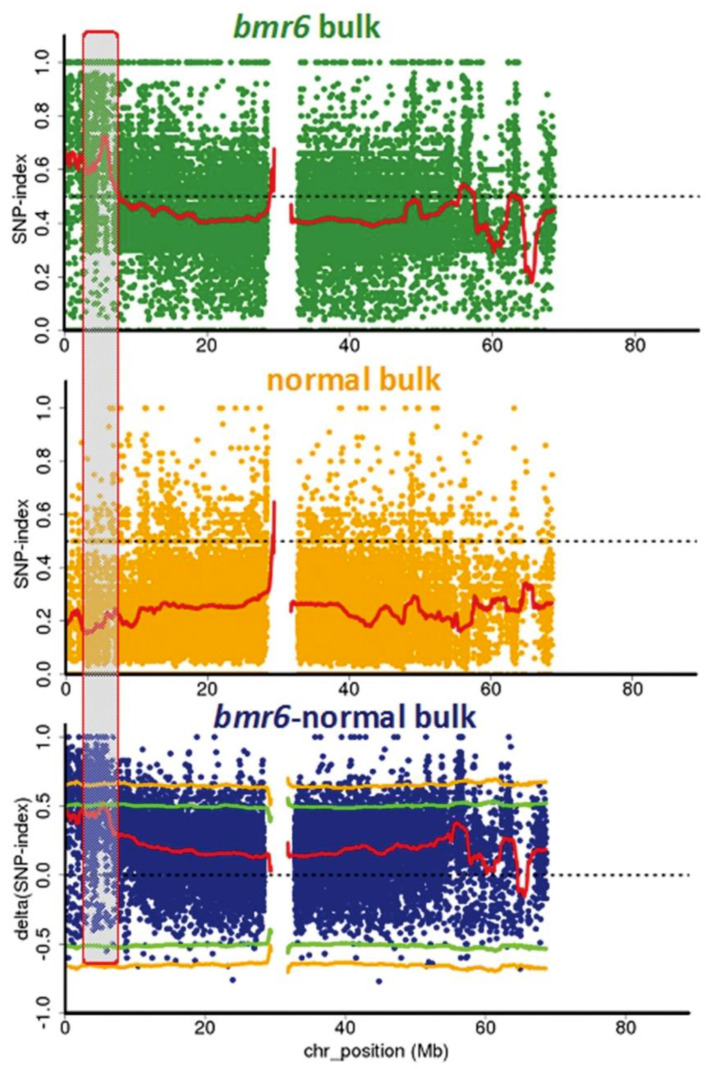
The aligned consensus assemblies of the bmr6 and normal bulks showed a SNP index peak in the genomic interval from 4Mb to 6.6 Mb on Chromosome 4. The analysis of SNPs in this candidate region revealed a candidate SNP corresponding to Sobic.004G071000, encoding CAD2. The presumed causal SNP at this locus between the bulks was at position Chr04:5,731,386 (Phytozome Sorghum bicolor v.3.1.1) with a C → T on the reverse strand, the same mutation defined as the bmr6-ref allele, resulting in a predicted nonsense mutation Q132stop [6]. The SNP indices plots for Chromosome 4 comparing the bmr6 and normal bulks are shown in Figure 2. The highest peak of the ΔSNP-index plot between the bmr6 and normal bulks defining this candidate region approached the p < 0.05 threshold, but like the bmr2 comparison, fell short of this significance level due to the presence in the bmr6 bulk of a second mutant allele, bmr6-3 contributed by brown midrib mutant line bmr3 in the pedigrees of two members of the bulk (Table 1). This allele, G → A on the reverse strand at position Chr04:5,730,502 predicted to cause the missense mutation G129S [6], was present at a low frequency among the aligned reads in the bmr6 bulk. Its frequency was low enough to prevent miscall of the consensus assembly comparison variation between the bmr6 and normal bulks in the analysis.
Figure 2.
Single nucleotide polymorphism (SNP) analysis to identify bmr6 mutation. SNP index plots of bmr6 bulk (top), normal bulk (middle) and Δ(SNP-index) plot (bottom) of sorghum Chromosome 4 with statistical confidence intervals under the null hypothesis of no QTLs (green, p < 0.05; yellow, p < 0.01). Grey highlighted area shows the QTL near the beginning of Chromosome 4 where the significant difference between the bulks occurs.
3.3. Comparison of the bmr12 and Normal Bulks
The analysis failed to pick a consistent difference between the bmr12 and normal bulks at the Bmr12 gene, Sobic.007G047300, encoding for COMT [21]. Therefore, no candidate genomic region was defined for this comparison of bulks at this locus. This was likely due to a mixture of mutant alleles in the bmr12 bulk and for variation among the members of the normal bulk for the indels in this gene described by Gorthy et al. [18]. We examined members of both the bmr12 and normal bulks by primers that amplified the regions containing these indels [36]. Examination of the aligned reads at this locus shows a high frequency of both the bmr12-18 (G → A on the reverse strand at position Chr07:4,724,050, at a frequency of 67%) and bmr12-ref (C → T on the reverse strand at position Chr07:4,723,997, at a frequency of 7%) defined by Bout and Vermerris [21] among the pooled reads of the bmr12 bulk. A few reads (frequency = 17%) in this bulk also showed the bmr12-7 allele [14], a C → T on the reverse strand at position Chr07:4,722,193.
3.4. Identification of the Bmr19 Gene
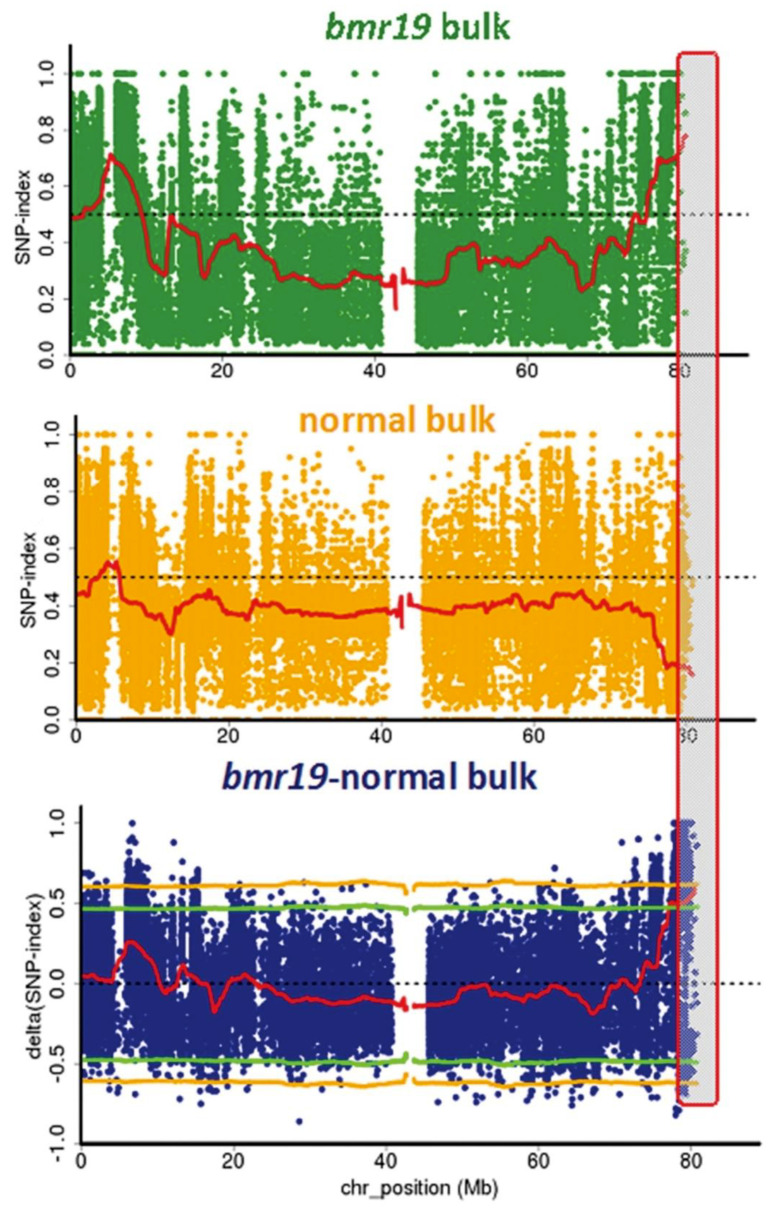
The same analysis performed on the other brown midrib bulks indicated a SNP-index peak in the genomic interval from 70 Mb to 80 Mb on at the end of sorghum Chromosome 1 when the bmr19 and normal bulks were compared (Figure 3). Analysis of SNPs in this candidate region revealed a candidate SNP G → A mutation on the forward strand at position Chr01:79,894,470 (Phytozome Sorghum bicolor v.3.1.1) in the predicted gene Sobic.001G535500. This gene encodes a putative folylpolyglutamate synthase (FPGS) with high homology (95.4% identical + positively scoring residues in alignment according to Phytozome v.12) to maize Bm4, GRMZM2G393334 [41]. The maize bm4 mutant has a brown midrib phenotype and, like bmr19, shows only slight changes in lignin amount and composition [41,42]. The mutation (bmr19-ref) defined by this SNP occurs in the seventh exon of the gene model (Phytozome Sorghum bicolor v3.1.1) and is predicted to cause the missense mutation G240E. The mutation occurs within the annotated Mur-ligase domain in a part associated with binding the glutamic acid. This mutation has changed the chemical characteristic of this amino acid residue from without a side chain (glycine) to one with a large side chain containing negative charge (glutamate) that could interfere with substrate binding and may affect folding the protein. There are no other variations at this position among the 71 resequenced sorghum lines in Phytozome v.12 [33,34]. This allele is present in all reads aligning to this position in the bmr19 bulk and absent in all reads of the normal bulk, which all have a G at this position.
Figure 3.
Single nucleotide polymorphism (SNP) analysis to identify bmr19 mutation. SNP index plots of bmr19 bulk (top), normal bulk (middle) and Δ(SNP-index) plot (bottom) of sorghum Chromosome 1 with statistical confidence intervals under the null hypothesis of no QTLs (green, p < 0.05; yellow, p < 0.01). Grey highlighted area shows the QTL at the end of Chromosome 1 where the significant difference between the bulks occurs.
3.5. Association of the bmr19-ref Allele with the Brown Midrib Phenotype Outside the Mapping Population
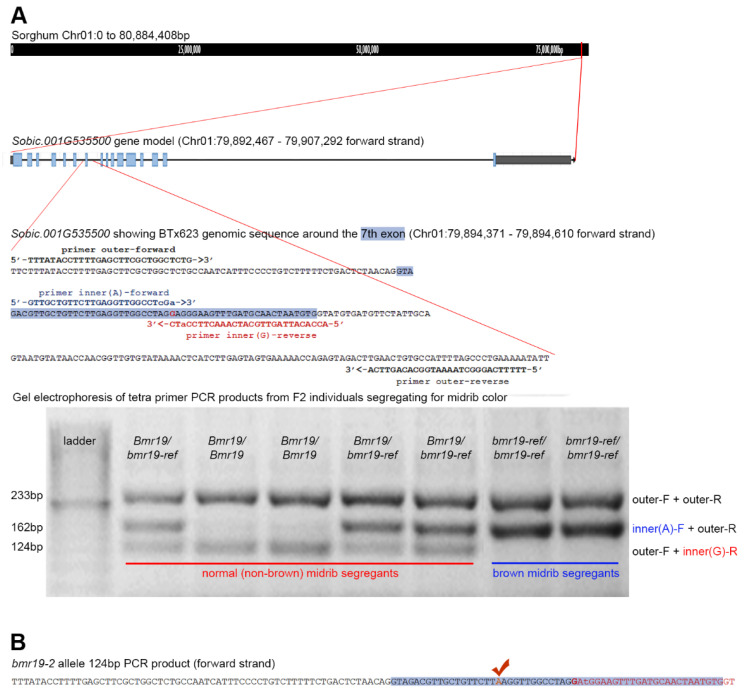
An allele specific primer set was designed to convert the SNP to a size polymorphism upon PCR amplification and gel electrophoresis (Figure 4). The bmr19-ref allele (A) was resolved from the wildtype allele (G) by 38 bp difference in product size. This marker was run against the nine lines constituting the normal bulk, which all showed G and the nine members of the bmr19 bulk which all gave amplicons corresponding to A at this position. The marker was also used on the original brown midrib mutant lines of Porter et al. [13] and these all, with the exception of bmr19 which had the A allele, gave bands corresponding to the wildtype allele. We also checked two F2 populations derived from the original bmr19 line, one from a cross with BTx623 and the second with IS4225, both with non-brown phenotypes. The marker representing the A allele always cosegregated with the visually scored brown midrib plants and the normal (non-brown midrib) segregants showed either the G allele or both a G and A allele, representing heterozygotes (Figure 4). The ratios of brown midrib to normal plants in the (BTx623 × bmr19)F2 fit a 3:1 ratio ( = 0.02; p = 0.889), with 35 progeny having the genotype bmr19-ref/bmr19-ref, 69 with Bmr19/bmr19-ref and 45 with Bmr19/Bmr19. In the (bmr19 × IS4225)F2 population, the number of brown midrib individuals also fit a 3:1 ratio ( = 0.095; p = 0.758), all the brown midrib mutants (69 plants) were homozygous for the A allele (bmr19-ref/bmr19-ref). This F2 population had 143 heterozygous individuals and 73 were homozygous for the wildtype allele (GG) consistent with the hypothesis that the bmr19 mutant phenotype is caused by a recessive mutation in a single locus. From these observations, G → A transition mutation in the bmr19-ref allele of Sobic.001G535500 on the forward strand at position Chr01:79,894,470 likely results in the brown midrib phenotype of the original bmr19 donor.
Figure 4.
Detection of alleles at Bmr19 using DNA based markers. (A) Location and scheme for the primer set used to detect the SNP distinguishing the bmr19-ref (A) from wildtype Bmr19 (G) alleles. Based on the method described by Medrano and de Oliveira (2014) [37], four primers in the PCR amplify three possible products. The outer primers (outer-F + outer-R) result in a control amplicon of 233 bp in all samples. Mismatches are introduced near the 3′ ends of both inner primers (small case) which both end on the SNP. In the PCR with a wildtype template allele (Bmr19), only the inner(G)-R primer works to give a second amplicon of 124 bp with outer-F since the second mismatch at the 3′ end of inner(A)-F is not tolerated. Only when there is an “A” at this position, as occurs in bmr19-ref, will the inner(A)-F give an amplicon with outer-R of 162 bp. Because of the double mismatch at the 3′ end, inner(G)-R does not work with the mutant template. The PCR products are separated by size with gel electrophoresis. In F2 populations derived from bmr19 crossed with wildtype that segregate for midrib phenotypes, all brown midrib individuals give two bands (233 + 162 bp) corresponding to the bmr19-ref/bmr19-ref genotype. Normal plants give either two bands (233 + 124 bp) if they are homozygous (Bmr19/Bmr19) or three bands (233 + 162 + 124 bp) if they are heterozygous (Bmr19/bmr19-ref). (B) Due to the proximity of the SNP distinguishing the bmr19-2 allele, the same tetra primer set was useful for confirming F1s from the allelism test cross of emasculated bmr19 with 2354. PCR products from true F1s (bmr19-ref/bmr19-2) give three bands like the heterozygotes pictured above while any selfs due to incomplete emasculation (bmr19-ref/bmr19-ref) give only two bands. Sequencing of the smallest band (124 bp) from F1 plants showed the bmr19-2 mutation G → A at the position indicated in orange. The genotype bmr19-ref/bmr19-2 had a brown midrib phenotype, confirming that the mutations are allelic.
3.6. Alternate Alleles
One of the four variants at this locus, which we named bmr19-2, also resulted in a brown midrib phenotype (Table 2). This line (2354) contains a G → A transition mutation at Chr01:79,894,457 resulting in the amino acid substitution E236K. Like bmr19-ref, this substitution occurs in the Mur-ligase domain. Here, the negatively charged glutamate is changed to a positively charged lysine residue, perhaps also interfering with substrate binding. The three other EMS derived lines from BTx623 contained missense mutations with substitutions of similar amino acids, even though these substitutions also occur in the Mur ligase domain. Line 0623 has a C → T substitution relative to the reference genome at Chr01:79,894,180 resulting in a predicted A221V substitution whereby a hydrophobic alanine residue is replaced with valine, also hydrophobic. In line 2465, a G → A at Chr01:79,895,561 changes the predicted 378th codon from one for serine, a polar residue, to one for asparagine which is also polar. Line 0662 has two variations in its annotated coding regions with respect to the reference. The first is a G → A at Chr01:79,894,460 causing a valine (nonpolar) to isoleucine (also nonpolar) amino acid substitution at the 237th residue of the predicted protein. The second is a synonymous G → A mutation at Chr01:79,896,564 at the 550th codon for an arginine residue. None of the variations at Sobic.001G535500 carried by EMS lines 0623, 2465 and 0662 result in brown midrib phenotypes. An additional natural variant allele was revealed in the sequence of sorghum lines Ajabsido (PI 656015; SRR2759170), Feterita Gishesh (PI 152651; SRR2759175), Koro-Kollo (PI 656065; SRR2759174) and Tx436 (PI 561071; SRR2759165). These share the common variation from the reference genome of C → A at Chr01:79,893,872 resulting in a serine (polar) to tyrosine (also polar) amino acid substitution at the 185th residue. Like the three innocuous EMS mutations, these variants do not have a brown midrib phenotype. The bmr19 bulk also shares this variation from the reference, but given the presence of this mutation in the normal bulk and by inference among the majority of the recurrent parents of the introgression lines, we ruled it out as the cause of the brown midrib phenotype.
The allelic nature of the causal mutations was confirmed by crossing 2354 onto the original bmr19 line. Since the female (bmr19) had to be emasculated to conduct the cross, the progeny was checked for self-pollination events resulting from incomplete emasculation with the same tetra primers used to track the bmr19-ref allele in the F2 populations described above and in Figure 4. All of the progeny showed the brown midrib phenotype. All the progenies were true F1s (bmr19-ref/bmr19-2) and these were distinguishable from any progeny resulting from self-pollination by the presence of a third PCR product of 124 bp. Confirmation of the bmr19-2 allele in the heterozygotes was achieved by sequencing this PCR product which showed an “A” at the position corresponding to Chr01:79,894,457 of the genomic template. The identification of a second mutant allele, bmr19-2, and the brown midrib phenotype associated with it in the homozygous state or in heterozygous combination with the bmr19-ref allele confirms the identity of Bmr19 as Sobic.001G535500.
3.7. Effects on Feedstock
In order to determine the effects of the bmr19 mutations on lignocellulosic quality of sorghum feedstock, we compared stover of the introgression line carrying the bmr19-ref allele [(BTx623ms3 × bmr19)BC2F4] with BTx623 and EMS line 2354 (BTx623 derivative carrying the bmr19-2 allele). Both mutant lines were slightly later in maturity than BTx623, but similar in plant architecture, distinguished mainly by the color of their midribs (Figure 5). The stover for fiber analysis was collected from all lines on the same date when all plants had reached maturity. Similar to earlier compositional analysis of the original bmr19 line [14], we found slight but significant reductions with respect to wildtype in lignin content of biomass in both BTx623 derivatives bearing bmr19 mutations (Table 3 and Table 4). These reductions are less severe than in BTx623 derivatives bearing bmr2, bmr6 or bmr12 mutations (Table 3). In terms of the lignin subunit yields from thioacidolysis, both bmr19 mutations cause a slight but significant increase in H-subunits, although these remain relatively minor constituents of lignin polymers in wildtype and mutant lines. Both G- and S-subunits are reduced in bmr19 mutants relative to wildtype. Reduction of the single methoxylated G-subunits, ranging from 16–25%, are approximately twice the reductions (7–14%) of the double methoxylated S-subunits, reflected in the lower G/S ratio of these mutants with respect to wildtype (Table 5). The changes in monomer content appear to be slightly more pronounced in the line bearing the bmr19-ref allele, particularly in the reduction of G-subunits.
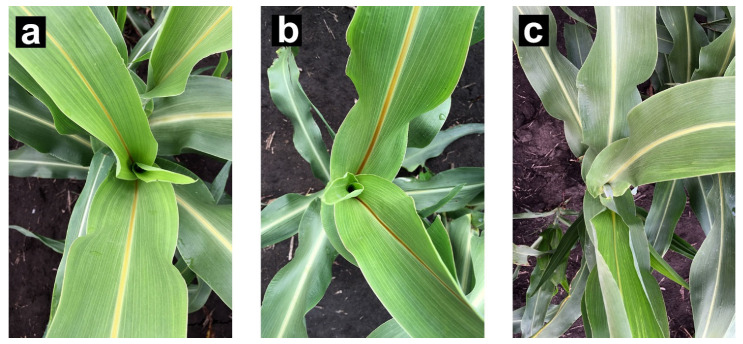
Figure 5.
Visible midrib phenotypes of bmr19 mutants and wildtype in the BTx623 background. Photos taken from field grown plants at early boot stage: (a) brown midrib line (BTx623ms3 × bmr19)BC2F4, carrying the bmr19-ref allele; (b) brown midrib BTx623 EMS line 2354, carrying the bmr19-2 allele; (c) normal midrib BTx623, carrying the wildtype (Bmr19) allele.
Table 3.
Fiber analysis of greenhouse grown wildtype and bmr introgression lines in the BTx623 background.
| Line | Relevant Alleles in Homozygosity | Neutral Detergent Fiber (Cellulose, Hemicellulose and Lignin) | Acid Detergent Fiber (Cellulose and Lignin) | Acid Detergent Lignin (Lignin) | |||
|---|---|---|---|---|---|---|---|
| Mean * | Std Dev | Mean | Std Dev | Mean | Std Dev | ||
| BTx623 | Bmr2, Bmr6, Bmr12, Bmr19 | 63.1 a | 5.7 | 37.5 a | 4.0 | 5.0 a | 0.6 |
| (bmr2ms3 × Tx623B)BC3F5 | bmr2-ref | 58.9 ab | 6.0 | 34.1 ab | 3.9 | 2.6 c | 0.4 |
| (bmr6ms3 × Tx623B)BC3F5 | bmr6-ref | 55.8 b | 8.1 | 31.8 b | 5.4 | 2.5 c | 0.3 |
| (BTx623ms3 × bmr18)BC2F4 | bmr12-18 | 57.6 b | 4.2 | 32.6 b | 2.7 | 2.5 c | 0.3 |
| (BTx623ms3 × bmr19)BC2F4 | bmr19-ref | 59.7 ab | 5.6 | 33.5 ab | 4.3 | 3.6 b | 0.2 |
| BTx623 EMS line 2354 | bmr19-2 | 62.2 ab | 3.5 | 34.8 ab | 2.5 | 3.7 b | 0.9 |
* Means with the same letter are not significantly different. Values expressed as g/kg dry weight.
Table 4.
Fiber analysis of field grown mutant (bmr19) and wild type (Bmr19) sorghum lines in the BTx623 background.
| Genotype * | Neutral Detergent Fiber (Cellulose, Hemicellulose and Lignin) | Acid Detergent Fiber (Cellulose and Lignin) | Acid Detergent Lignin (Lignin) | |||
|---|---|---|---|---|---|---|
| Mean † | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| Bmr19 | 55.8 b | 1.7 | 29.1 b | 1.0 | 4.6 a | 0.2 |
| bmr19-ref | 56.1 b | 2.2 | 28.2 b | 1.2 | 3.9 c | 0.2 |
| bmr19-2 | 61.9 a | 1.8 | 31.8 a | 1.5 | 4.3 b | 0.2 |
* Bmr19 = wildtype allele in homozygosity in BTx623; bmr19-ref = mutant allele in homozygosity in (BTx623ms3 × bmr19)BC2F4; bmr19-2 = mutant allele in homozygosity in line 2354. † Means with the same letter are not significantly different. Values expressed as g/kg dry weight.
Table 5.
Lignin monomer [p-hydroxyphenyl (H), syringyl (S), guaiacyl (G)] yield by thioacidolysis of field grown biomass from BTx623 and derivatives.
| Genotye * | H (µg/g Dry Weight) | G (µg/g Dry Weight) | S (µg/g Dry Weight) | G/S | ||||
|---|---|---|---|---|---|---|---|---|
| Mean † | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| Bmr19 | 6607.1 a | 852.3 | 169,109.5 a | 25,095.0 | 104,810.2 a | 21,144.7 | 1.6 a | 0.1 |
| bmr19-ref | 7629.6 b | 1134.5 | 126,173.5 c | 17,264.5 | 89,806.9 b | 16,331.9 | 1.4 b | 0.1 |
| bmr19-2 | 7239.5 ab | 951.1 | 141,872.7 b | 15,806.5 | 97,702.2 ab | 12,935.2 | 1.5 b | 0.1 |
* Bmr19 = wildtype allele in homozygosity in BTx623; bmr19-ref = mutant allele in homozygosity in (BTx623ms3 × bmr19)BC2F4; bmr19-2 = mutant allele in homozygosity in line 2354. † Means with the same letter are not significantly different.
4. Discussion
4.1. Power of the Analysis of Whole Genome Resequenced Bulk Comparisons
Whole genome resequencing of bulked DNA from near inbred lines (most at F4 or F5) carrying particular brown midrib phenotypes compared to bulked DNA from wildtype parents in the original crosses successfully distinguished the causal mutant and wildtype alleles for these phenotypes when the donor mutant alleles were shared among the members of the bmr bulks. This occurred for the bmr2-ref allele and bmr6-ref allele, despite the “contamination” of these mutant bulks with alternate alleles. Mixed alleles undermined detection of a candidate SNP-index peaks by the analytical protocol employed in the bmr12 bulk because the donor mutant alleles were more evenly mixed with five of the nine members of the bmr12 bulk having the bmr12-18 allele, three with the bmr12-ref allele and one with the bmr12-7 allele (Table 1). Although the analysis failed to pick a common polymorphism between the bmr12 and normal bulks in the aligned consensus sequence, the presence of all these mutant alleles among the reads aligning to this locus can be seen using IGV software [35] in the bmr12 bulk. The analysis was further hindered by polymorphisms among the members of the normal bulk of functionally inert indels in the Bmr12 gene. Recurrent parents Sudan Zera-Zera and P9401 share these polymorphisms (missing Olo24 and two repeat units in the SSR) with the bmr7, bmr12 and bmr18 donor parents. Because the analysis filtered the consensus assembly by comparison to Sudan Zera-Zera, these polymorphisms skewed the results in this region.
4.2. The Bmr19 Locus
The analysis showed a genomic interval with a SNP-index peak between the bmr19 and normal bulks and within this candidate genomic interval at Sobic.001G535500, a single SNP that is strongly suggested to be the causal mutation. This bmr19-ref allele represents a missense mutation G240E occurring within the annotated Mur-ligase domain associated with binding the glutamic acid substrate. All brown midrib introgression lines derived from the original bmr19 line contain this variation and the mutation always cosegregated with the brown midrib phenotype in F2 populations. An alternate allele at this locus confirmed the identity of Sobic.001G535500 as Bmr19. The E236K mutation in the brown midrib EMS line 2354 carrying the bmr19-2 allele (Table 2) similarly affects a residue charge in the Mur ligase domain of the gene product. Less severe amino acid substitutions in which the charge characteristics of the residues remain unchanged in this domain did not cause a mutant phenotype.
The predicted product of Sobic.001G535500 or Bmr19 is a FPGS, an enzyme involved in folate (C1) metabolism. Its link to lignin biosynthesis is best explained in two publications describing the maize bm4 mutant [41] and the fpgs1 mutant of Arabidopsis [43]. Briefly, the tie of C1 metabolism to lignin is that it synthesizes the methyl donor, SAM, that COMT and CCoAOMT use to form the methoxy groups of coniferyl and sinapyl alcohols which ultimately become the G and S subunits of the lignin polymer. The actual role of FPGS in the biosynthesis of SAM is to add glutamic acid to tetrahydrofolate (THF) to form a short polyglutamate tail important for efficient and properly compartmentalized function of THF [44] in its role as cofactor in the downstream methylation of homocysteine to methionine, a direct precursor of SAM. Both polyglutamylated folates and SAM are vital in the biosynthesis of several primary and secondary plant metabolites, including purines, the amino acids methionine and serine and betaines [45], so a complete loss of FPGS activity would likely be lethal. A second predicted FPGS (Sobic.001G201900) is present in the sorghum genome, which may be sufficiently redundant to prevent these deleterious effects. One might expect that the mutations in bmr19-ref and bmr19-2 impair lignin biosynthesis by limiting the supply of the cofactor SAM to the O-methyltransferases in the pathway thereby impairing the synthesis of coniferyl and sinapyl monolignols. Our analysis of lignin composition via thioacidolysis in bmr19 stover showed that S-subunits were less reduced than G-subunits with respect to wildtype. A decreased G:S ratio of lignins was also reported in maize bm4 mutant stalks [41,42,46]. This suggests that the C1 metabolites feeding the guaiacyl branch of the lignin biosynthetic pathway are disproportionately limited. In Arabidopsis, expression of other genes in the lignin biosynthetic pathway was reportedly downregulated in fpgs1 mutants, implying some feedback control that rations the limited C1 metabolites, including SAM, to cellular functions more vital than lignification [43]. The introgression lines carrying the bmr19-ref mutation will be excellent materials in which to monitor any possible regulatory effects on other lignin biosynthetic genes in sorghum.
Considering the relatively minor effects of bmr19 alleles on lignocellulosic properties of sorghum with respect to bmr6 and bmr12, these mutants at first seem not to be as useful as the other bmr mutations to improving saccharification of feedstock for biofuel production. Future work may generate stronger bmr19 alleles that more severely impair or eliminate this specific FPGS product feeding sorghum lignin biosynthesis with possible improvements to feedstock quality.
A FPGS in switchgrass (Panicum virgatum) encoded by Pavir.Ib00114 (PvFPGS1) with high homology (93.8% identical + positively scoring residues in alignment according to Phytozome v.12) to sorghum Bmr19 was targeted by RNAi to either knock-down or knock-out the activity of one of three FPGS in transgenic plants evaluated in the field for harvestable biomass and ethanol yield [47]. Transgenic lines in which PvFPGS1 expression was essentially knocked out had severe consequences on plant biomass yield. Similar to the current analyses of bmr19 sorghum mutants, lignin content and composition of moderately knocked-down PvFPGS1 transgenic switchgrass was little changed from controls. However, in certain of these transgenic switchgrass lines ethanol yield was improved by as much as 18%. This was despite no obvious improvement in sugar release efficiency in these transgenics relative to control [47]. The authors of the switchgrass study monitored global gene expression in their transgenic lines through RNA-seq and speculate that the increased ethanol yield in their low-to-moderate PvFPGS1 knock-down transgenics may be due to perturbations in gene expression through metabolite feedback that alter the expression of genes whose products ultimately affect the level of lignin polymerization in cell walls rather than the actual production of monolignols [47].
Having identified the Bmr19 locus as encoding an enzyme peripheral to the canonical monolignol biosynthetic pathway, the metabolic targets for engineering improved lignocellulosic feedstock from sorghum has expanded. Improvements to ethanol yield from sorghum bearing the bmr19 mutations described herein may not be great but other alleles at this locus that more severely affect FPGS function may be more useful. Plants with both the fpgs1 and ccoaomt1 mutations in Arabidopsis increased the enzymatic polysaccharide hydrolysis efficiencies over those of single mutants by up to 20% without adverse growth effects [48]. Similar double mutants like bmr12bmr19 bearing sorghum could result in better ethanol yields.
Author Contributions
Conceptualization, A.O.A., P.J.R. and G.E.; Data curation, A.O.A.; Formal analysis, A.O.A.; Funding acquisition, G.E.; Investigation, A.O.A., S.E.S., P.J.R., L.A.R.-B. and X.X.; Methodology, A.O.A., S.E.S., P.J.R. and L.A.R.-B.; Project administration, P.J.R. and G.E.; Supervision, G.E.; Validation, A.O.A. and P.J.R.; Visualization, A.O.A. and P.J.R.; Writing—original draft, A.O.A. and P.J.R.; Writing—review & editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Sorghum & Millet Innovation Lab (SMIL), through a grant from the United States Agency for International Development’s Bureau of Food Security.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study, are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cesarino I., Simões M.S., dos Santos Brito M., Fanelli A., da Franca Silva T., Romanel E. Building the wall: Recent advances in understanding lignin metabolism in grasses. Acta Physiol. Plant. 2016;38:269–282. doi: 10.1007/s11738-016-2293-5. [DOI] [Google Scholar]
- 2.Hatfield R.D., Marita J.M., Hatfield R.D., Marita J.M. Maize development: Cell wall changes in leaves and sheaths. Am. J Plant Sci. 2017;8:1248–1263. doi: 10.4236/ajps.2017.86083. [DOI] [Google Scholar]
- 3.Li H., Huang Y. Expression of brown-midrib in a spontaneous sorghum mutant is linked to a 5′-UTR deletion in lignin biosynthesis gene SbCAD2. Sci. Rep. 2017;7:11664. doi: 10.1038/s41598-017-10119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Börjesson P., Tufvesson L.M. Agricultural crop-based biofuels—Resource efficiency and environmental performance including direct land use changes. J. Clean. Prod. 2011;19:108–120. doi: 10.1016/j.jclepro.2010.01.001. [DOI] [Google Scholar]
- 5.Li X., Zheng Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017;35:466–489. doi: 10.1016/j.biotechadv.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Saballos A., Ejeta G., Sanchez E., Kang C., Vermerris W. A genomewide analysis of the cinnamyl alcohol dehydrogenase family in sorghum [Sorghum bicolor (L.) Moench] identifies SbCAD2 as the brown midrib6 gene. Genetics. 2009;181:783–795. doi: 10.1534/genetics.108.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saballos A., Sattler S.E., Sanchez E., Foster T.P., Xin Z., Kang C., Pedersen J.F., Vermerris W. Brown midrib2 (Bmr2) encodes the major 4-coumarate: Coenzyme A ligase involved in lignin biosynthesis in sorghum (Sorghum bicolor (L.) Moench) Plant J. 2012;70:818–830. doi: 10.1111/j.1365-313X.2012.04933.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen B., Li C., Tarczynski M.C. High free-methionine and decreased lignin content result from a mutation in the Arabidopsis S-adenosyl-L-methionine synthetase 3 gene. Plant J. 2002;29:371–380. doi: 10.1046/j.1365-313X.2002.01221.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Chantreau M., Sibout R., Hawkins S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013;4:220. doi: 10.3389/fpls.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattler S.E., Funnell-Harris D.L., Pedersen J.F. Brown midrib mutations and their importance to the utilization of maize, sorghum, and pearl millet lignocellulosic tissues. Plant Sci. 2010;178:229–238. doi: 10.1016/j.plantsci.2010.01.001. [DOI] [Google Scholar]
- 11.Umezawa T., Sakamoto M., Koshiba T. Cinnamyl alcohol dehydrogenase deficiency causes the brown midrib phenotype in rice. In: Kumar A., Ogita S., Yau Y., editors. Biofuels: Greenhouse Gas Mitigation and Global Warming. Springer; New Delhi, India: 2018. pp. 375–385. [Google Scholar]
- 12.Koshiba T., Murakami S., Hattori T., Mukai M., Takahashi A., Miyao A., Hirochika H., Suzuki S., Sakamoto M., Umezawa T. CAD2 deficiency causes both brown midrib and gold hull and internode phenotypes in Oryza sativa L. cv. Nipponbare. Plant Biotechnol. 2013;30:365–373. doi: 10.5511/plantbiotechnology.13.0527a. [DOI] [Google Scholar]
- 13.Porter K.S., Axtell J.D., Lechtenberg V.L., Colenbrander V.F. Phenotype, fiber composition, and in vitro dry matter disappearance of chemically induced brown midrib (bmr) mutants of sorghum. Crop. Sci. 1978;18:205–208. doi: 10.2135/cropsci1978.0011183X001800020002x. [DOI] [Google Scholar]
- 14.Saballos A., Vermerris W., Rivera L., Ejeta G. Allelic association, chemical characterization and saccharification properties of brown midrib mutants of sorghum (Sorghum bicolor (L.) Moench) BioEnergy Res. 2008;1:193–204. doi: 10.1007/s12155-008-9025-7. [DOI] [Google Scholar]
- 15.Sattler S.E., Saballos A., Xin Z., Funnell-Harris D.L., Vermerris W., Pedersen J.F. Characterization of novel sorghum brown midrib mutants from an EMS-mutagenized population. G3. 2014;4:2115–2124. doi: 10.1534/g3.114.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpin C., Holt K., Chojecki J., Oliver D., Chabbert B., Monties B., Edwards K., Barakate A., Foxon G.A. Brown-midrib maize (bm1)—A mutation affecting the cinnamyl alcohol dehydrogenase gene. Plant J. 1998;14:545–553. doi: 10.1046/j.1365-313X.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- 17.Sattler S.E., Saathoff A.J., Haas E.J., Palmer N.A., Funnell-Harris D.L., Sarath G., Pedersen J.F. A nonsense mutation in a cinnamyl alcohol dehydrogenase gene is responsible for the sorghum brown midrib6 phenotype. Plant Physiol. 2009;150:584–595. doi: 10.1104/pp.109.136408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorthy S., Mayandi K., Faldu D., Dalal M. Molecular characterization of allelic variation in spontaneous brown midrib mutants of sorghum (Sorghum bicolor (L.) Moench) Mol. Breed. 2013;31:795–803. doi: 10.1007/s11032-012-9834-4. [DOI] [Google Scholar]
- 19.Scully E.D., Gries T., Funnell-Harris D.L., Xin Z., Kovacs F.A., Vermerris W., Sattler S.E. Characterization of novel Brown midrib 6 mutations affecting lignin biosynthesis in sorghum. J. Integr. Plant Biol. 2016;58:136–149. doi: 10.1111/jipb.12375. [DOI] [PubMed] [Google Scholar]
- 20.Dien B.S., Sarath G., Pedersen J.F., Sattler S.E., Chen H., Funnell-Harris D.L., Nichols N.N., Cotta M.A. Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. BioEnergy Res. 2009;2:153–164. doi: 10.1007/s12155-009-9041-2. [DOI] [Google Scholar]
- 21.Bout S., Vermerris W. A candidate-gene approach to clone the sorghum Brown midrib gene encoding caffeic acid O-methyltransferase. Mol. Gen. Genet. 2003;269:205–214. doi: 10.1007/s00438-003-0824-4. [DOI] [PubMed] [Google Scholar]
- 22.Vignols F., Rlgau J., Torres M.A., Capellades M., Puigdomènech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell. 1995;7:407–416. doi: 10.1105/tpc.7.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bittinger T.S., Cantrell R.P., Axtell J.D. Allelism tests of the brown- midrib mutants of sorghum. J. Hered. 1981;72:147–148. doi: 10.1093/oxfordjournals.jhered.a109455. [DOI] [Google Scholar]
- 24.Xin Z., Wang M.L., Burow G., Burke J. An induced sorghum mutant population suitable for bioenergy research. BioEnergy Res. 2009;2:10–16. doi: 10.1007/s12155-008-9029-3. [DOI] [Google Scholar]
- 25.Sattler S., Palmer N., Saballos A., Greene A.M., Xin Z., Sarath G., Vermerris W., Pedersen J.F. Identification and characterization of four missense mutations in Brown midrib 12 (Bmr12), the caffeic O-methyltranferase (COMT) of sorghum. BioEnergy Res. 2012;5:855–865. doi: 10.1007/s12155-012-9197-z. [DOI] [Google Scholar]
- 26.Eudes A., Dutta T., Deng K., Jacquet N., Sinha A., Benites V.T., Baidoo E.E., Richel A., Sattler S.E., Northen T.R., et al. SbCOMT (Bmr12) is involved in the biosynthesis of tricin-lignin in sorghum. PLoS ONE. 2017;12:e0178160. doi: 10.1371/journal.pone.0178160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosugi S., Natsume S., Yoshida K., MacLean D., Cano L., Kamoun S., Terauchi R. Coval: Improving alignment quality and variant calling accuracy for next-generation sequencing data. PLoS ONE. 2013;8:e75402. doi: 10.1371/journal.pone.0075402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe A., Kosugi S., Yoshida K., Natsume S., Takagi H., Kanzaki H., Matsumura H., Yoshida K., Mitsuoka C., Tamiru M., et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012;30:174–178. doi: 10.1038/nbt.2095. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence M., Huber W., Pages H., Aboyoun P., Carlson M., Gentleman R., Morgan M.T., Carey V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obenchain V., Lawrence M., Carey V., Gogarten S., Shannon P., Morgan M. VariantAnnotation: A Bioconductor package for exploration and annotation of genetic variants. Bioinformatics. 2014;30:2076–2078. doi: 10.1093/bioinformatics/btu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mace E.S., Tai S., Gilding E.K., Li Y., Prentis P.J., Bian L., Cruickshank A., Campbell B.C., Hu W., Innes D.J., et al. Whole-genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum. Nat. Commun. 2013;4:2320. doi: 10.1038/ncomms3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paterson A.H., Bowers J.E., Bruggmann R., Grimwood J., Gundlach H., Haberer G., Hellsten U., Mitros T., Poliakov A., Schmutz J., et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2008;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 35.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative Genomics Viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera Burgos L.A. Ph.D. Dissertation. Purdue University; West Lafayette, IN, USA: 2015. Genetic, Agronomic and Compositional Characterization of Brown Midrib Sweet Sorghum Lignocellulosic Biomass for Ethanol Production. [Google Scholar]
- 37.Medrano R.F., de Oliveira C.A. Guidelines for the tetra-primer ARMS-PCR technique development. Mol. Biotechnol. 2014;56:599–608. doi: 10.1007/s12033-014-9734-4. [DOI] [PubMed] [Google Scholar]
- 38.Vogel K.P., Pedersen J.F., Masterson S.D., Toy J.J. Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop. Sci. 1999;39:276–279. doi: 10.2135/cropsci1999.0011183X003900010042x. [DOI] [Google Scholar]
- 39.Sarath G., Akin D.E., Mitchell R.B., Vogel K.P. Cell-wall composition and accessibility to hydrolytic enzymes is differentially altered in divergently bred switchgrass (Panicum virgatum L.) genotypes. Appl. Biochem. Biotechnol. 2008;150:1–14. doi: 10.1007/s12010-008-8168-5. [DOI] [PubMed] [Google Scholar]
- 40.Palmer N.A., Sattler S.E., Saathoff A.J., Funnell D., Pedersen J.F., Sarath G. Genetic background impacts soluble and cell wall-bound aromatics in brown midrib mutants of sorghum. Planta. 2008;229:115–127. doi: 10.1007/s00425-008-0814-1. [DOI] [PubMed] [Google Scholar]
- 41.Li L., Hill-Skinner S., Liu S., Beuchle D., Tang H.M., Yeh C.T., Nettleton D., Schnable P.S. The maize brown midrib4 (bm4) gene encodes a functional folylpolyglutamate synthase. Plant J. 2015;81:493–504. doi: 10.1111/tpj.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marita J.M., Vermerris W., Ralph J., Hatfield R.D. Variations in the cell wall composition of maize brown midrib mutants. J. Agric. Food Chem. 2003;51:1313–1321. doi: 10.1021/jf0260592. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava A.C., Chen F., Ray T., Pattathil S., Peña M.J., Avci U., Li H., Huhman D.V., Backe J., Urbanowicz B., et al. Loss of function of folylpolyglutamate synthetase 1 reduces lignin content and improves cell wall digestibility in Arabidopsis. Biotechnol. Biofuels. 2015;8:224. doi: 10.1186/s13068-015-0403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehrshahi P., Gonzalez-Jorge S., Akhtar T.A., Ward J.L., Santoyo-Castelazo A., Marcus S.E., Lara-Núñez A., Ravanel S., Hawkins N.D., Beale M.H., et al. Functional analysis of folate polyglutamylation and its essential role in plant metabolism and development. Plant J. 2010;64:267–279. doi: 10.1111/j.1365-313X.2010.04336.x. [DOI] [PubMed] [Google Scholar]
- 45.Hanson A.D., Roje S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:119–137. doi: 10.1146/annurev.arplant.52.1.119. [DOI] [PubMed] [Google Scholar]
- 46.Barrière Y., Ralph J., Méchin V., Guillaumie S., Grabber J.H., Argillier O., Chabbert B., Lapierre C. Genetic and molecular basis of grass cell wall biosynthesis and degradability. II. Lessons from brown-midrib mutants. Comptes Rendus Biol. 2004;327:847–860. doi: 10.1016/j.crvi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Mazarei M., Baxter H.L., Srivastava A., Li G., Xie H., Dumitrache A., Rodriguez M., Jr., Natzke J.M., Zhang J.Y., Turner G.B., et al. Silencing folylpolyglutamate synthetase1 (FPGS1) in switchgrass (Panicum virgatum L.) improves lignocellulosic biofuel production. Front. Plant Sci. 2020;11:843. doi: 10.3389/fpls.2020.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie H., Engle N.L., Venketachalam S., Yoo C.G., Barros J., Lecoultre M., Howard N., Li G., Sun L., Srivastava A.C., et al. Combining loss of function of FOLYLPOLYGLUTAMATE SYNTHETASE1 and CAFFEOYL-COA 3-O-METHYLTRANSFERASE1 for lignin reduction and improved saccharification efficiency in Arabidopsis thaliana. Biotechnol. Biofuels. 2019;12:108–127. doi: 10.1186/s13068-019-1446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study, are contained within the article.