Figure 3.
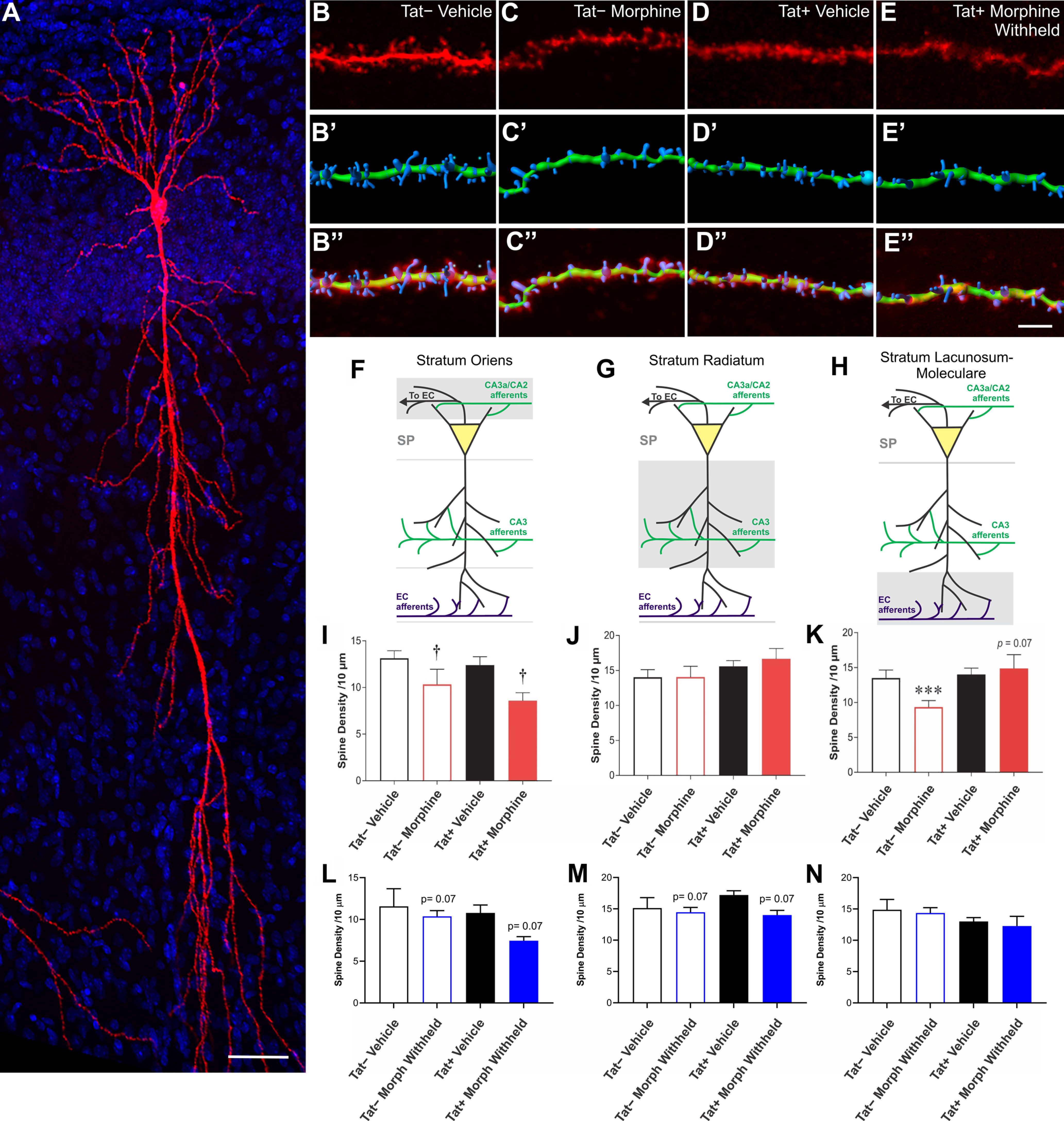
Effects of Tat and morphine on spine density along specific pyramidal cell dendritic segments within the SO, SR, and SL-M of hippocampal area CA1. A, Reconstructed Z-stack image of a representative biocytin-filled pyramidal cell within hippocampal area CA1 from a vehicle-treated Tat− mouse. Scale bar: 50 μm. B–E, Sample pyramidal cell dendritic segments and 3D reconstructions from the SO of control, morphine, Tat, and morphine plus Tat-exposed mice. The top image is the raw image file (B–E). The image below shows a representative 3D-reconstructed dendritic segment (green) and its associated spines (blue; B’–E’). The final image superimposes the raw image of the dendrite and its spines with the 3D reconstruction of the same dendritic segment and spines (B’’–E’’). Scale bar: 10 μm. F, I, L, Illustration of the location of pyramidal cell dendritic portion sampled is shaded in gray (F) and mean spine densities ± the SEM within the SO (I, L). I, There was a main effect of sustained morphine exposure in vivo and during recordings to reduce overall SO spine density (†p = 0.007). L, Spine losses were no longer significant after morphine was withheld, suggesting morphine-dependent spine losses are plastic and reversible. G, J, M, Illustration of the location of pyramidal cell dendritic portion sampled is shaded in gray (G) and mean spine densities ± the SEM within the SR (J, M). J, No differences in spine density in SR dendrites were observed in Tat− or Tat+ in the absence or presence of sustained exposure to morphine in vivo and during recordings ex vivo. M, By contrast, withholding morphine tended to induce spine losses. H, K, N, Illustration of the location of pyramidal cell dendritic portion sampled is shaded in gray (H) and mean spine densities ± the SEM within the SL-M (K, N). K, Morphine caused a reduction in SL-M dendritic spine density in pyramidal cells from Tat−, but not Tat+, mice (***p < 0.05). In fact, there was a trend for Tat to reverse morphine-dependent reductions in dendritic spine density with sustained morphine exposure ex vivo, albeit not significantly (p = 0.07), suggesting that Tat and morphine uniquely interact to increase spine numbers on the SL-M (G). N, By contrast, withholding morphine from ex vivo slices during recordings negated any changes in spine density seen when morphine is present (N vs K), suggesting the changes in spine density caused by morphine are highly plastic and modifiable.
