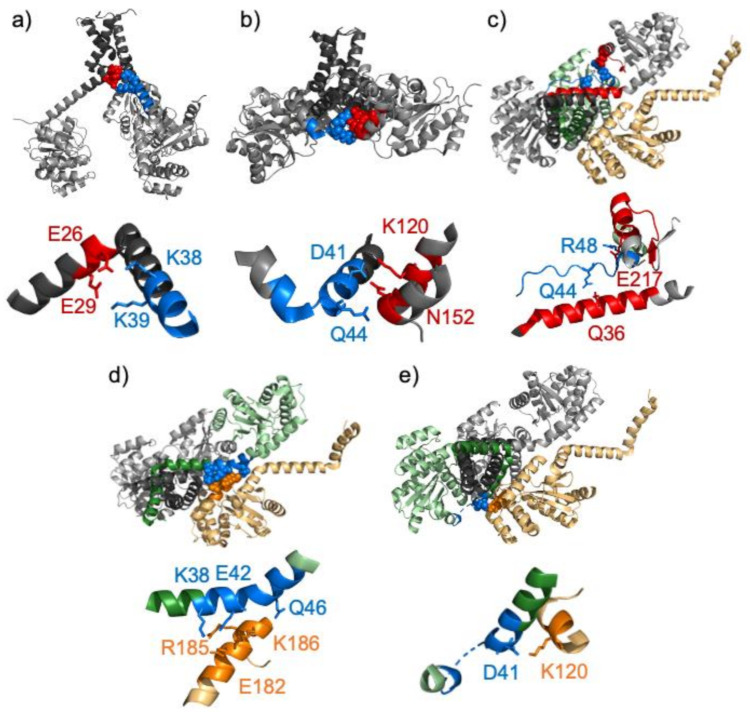
Figure 1.
Crystal structures of the PmScsC trimer show several different relative orientations of the trimerization stalk and the catalytic domains and different conformations for the eleven-residue linker peptide. The three molecules which form the trimer are shown in grey with the trimerization stalk shown in a darker grey. Contacts between molecules involving residues within the linker peptide region are shown in the full protein structures and in zoomed-in expansions with important residues shown as spheres and sticks, respectively. (a) The extended crystal structure (5id4). The linker peptide sequence is shown in blue in molecule A. Lys 38 and Lys39 make contacts to Glu 26 and Glu 29 in molecule B which are shown in red. (b) The compact crystal structure (4xvw). Molecules A, B and F are shown. The linker peptide sequence is shown in blue in molecule A. Asp 41 and Gln 44, shown in blue, make contacts to Lys 120 and Asn 152 in molecule F, shown in red. (c–e) The transitional crystal structure (5idr). Molecules A, B and C which form a trimer are shown; for clarity, molecules A, B or C are shown in pale green (with the trimerization stalk shown in dark green) in panels d, c or e, respectively. Molecule D from a neighbouring trimer is shown in yellow. In panel c the linker peptide sequence in molecule B is shown in blue and residues 25–44 and 200–217 in molecule A, with which it makes intermolecular contacts, are shown in red. Hydrogen bonds are formed between Gln 44 and Arg 48 on molecule B and Gln 36 and Glu 217 in molecule A. In panel d the linker peptide sequence in molecule A is shown in blue with Lys 38, Glu 42 and Gln 46 highlighted. These three residues make hydrogen bonds to Glu 182, Arg 185 and Lys 186 in molecule D which are shown in orange. In panel e the linker peptide sequence in molecule C is shown in blue and residues 117–120 in molecule D with which it makes intermolecular contacts are shown in orange. There is a salt bridge between Asp 41 in chain C and Lys 120 in molecule D. Note that the coordinates of residues 46–47 are missing from chain C.