Abstract
Trichothecium roseum is an important postharvest pathogen, belonging to an alkalizing group of pathogens secreting ammonia during fungal growth and colonization of apple fruits. Fungal pH modulation is usually considered a factor for improving fungal gene expression, contributing to its pathogenicity. However, the effects of inoculation with T. roseum spore suspensions at increasing pH levels from pH 3 up to pH 7, on the reactive oxygen species (ROS) production and scavenging capability of the apple fruits, affecting host susceptibility, indicate that the pH regulation by the pathogens also affects host response and may contribute to colonization. The present results indicate that the inoculation of T. roseum spores at pH 3 caused the lowest cell membrane permeability, and reduced malondialdehyde content, NADPH oxidases activity, O2●− and H2O2 production in the colonized fruit. Observations of the colonized area on the 9th day after inoculation at pH 3, showed that the rate of O2●− production and H2O2 content was reduced by 57% and 25%, compared to their activities at pH 7. In contrast, antioxidative activities of superoxide dismutase, catalase and peroxidases of fruit tissue inoculated with spores’ suspension in the presence of a solution at pH 3.0 showed their highest activity. The catalase and peroxidases activities in the colonized tissue at pH 3 were higher by almost 58% and 55.9%, respectively, on the 6th day after inoculation compared to inoculation at pH 7. The activities of key enzymes of the ascorbate-glutathione (AsA-GSH) cycle and their substrates and products by the 9th day after fruit inoculation at pH 3 showed 150%, 31%, 16%, and 110% higher activities of ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase and glutathione reductase, respectively, compared to pH 7. A similar pattern of response was also observed in the accumulation of ascorbic acid and dehydroascorbate which showed a higher accumulation at pH 3 compared to the colonization at pH 7. The present results indicate that the metabolic regulation of the pH environment by the T. roseum not only modulates the fungal pathogenicity factors reported before, but it induces metabolic host changes contributing both together to fungal colonization.
Keywords: Trichothecium roseum, environmental pH, reactive oxygen species, ascorbate-glutathione cycle
1. Introduction
Pathogens can dynamically alter the local pH at an infection site to suit the increasing expression of pathogenicity factors and the enzymatic arsenal that contribute to pathogenicity [1]. The ability to modify pH may be expressed in either direction, and fungi that raise or reduce it are described as ‘alkalizing fungi’ or ‘acidifying fungi’, respectively. Ambient alkalization by fungi is achieved by active secretion of ammonia, which is produced as a result of protease activity and deamination of amino acids. Other postharvest pathogens, such as Penicillium expansum, Penicillium digitatum, Penicillium italicum [2], Botrytis cinerea [3], and Sclerotinia sclerotiorum [4,5], utilize tissue acidification by organic acids to support their attack. Trichothecium roseum is an important postharvest pathogen with a wide range of fruit hosts [6] such as apples, pears, muskmelons, apricots, tomatoes, and other fruit [6]. It was recently reported to belong to the alkalinizing fungus group, which can activate various extracellular enzymes in lesions in a neutral or slightly alkaline environment [7].
pH modulation is considered a main mechanism for differentially activating pathogenicity factors in the pathogen but the effect of the modulation of pH by the fungus on the host response has not been reported. Reactive oxygen species (ROS) is an important pathogenicity factor of pathogens [8]. Overproduction of ROS in the host causes lipid peroxidation and damage of the cell membrane in the early stages of infection, leading to the reduction of host disease resistance [8]. Bao et al. (2014) found that Fusarium sulphureum with stronger pathogenicity not only led to higher ROS accumulation in potato tubers, but also weakened the ROS scavenging ability by inhibiting the activities of antioxidant enzymes and the ascorbate-glutathione (AsA-GSH) cycle [9]. Furthermore, Botrytis cinerea was able to enhance its pathogenicity in tomato leaves by decreasing the enzyme activities of the AsA-GSH cycle, suggesting the importance of the antioxidant mechanism regulation during fungal pathogenicity [10].
Studies have shown that environmental pH can regulate the production and scavenging of ROS in plants. When bean cells were transferred from low pH (5) to pH 7.2 buffer medium, the activities of NADPH oxidases, resulted in a rapid production of H2O2 [11]. In addition, in a condition of pH 4.0 an increasing activity of the AsA-GSH cycle, reduction of H2O2 content, and inhibition of membrane lipid peroxidation was observed in ginger seedlings [12]. In conditions where muskmelon tissue was treated with oxalic acid at pH 1.8, and increased the activity of the ASA-GSH cycle, this induced the expression of CmPOD, inhibited O2●− and H2O2 accumulation, and a delayed membrane lipid peroxidation, and increased cell membrane integrity [13]. These studies also showed that ammonia altered the K+/H+ concentration of intracellular and extracellular cells and promoted Ca2+ influx [14]. Ethylene glycol-bis-(b-amino-ethyl ether) N, N, N′, N′-tetraacetic acid (EGTA) treatment reduced the intracellular Ca2+ concentration of potato tubers and inhibited the expression of plasma membrane NADPH oxidases and NADPH oxidases activity, thereby reducing the production of ROS, which could be restored by adding exogenous Ca2+ [15]. In another host pathogen system Colletotrichum coccodes increased the pH of wounded tomato fruit by secreting ammonia, resulting in the activation of NADPH oxidases activity, and the induction of H2O2 synthesis, thereby resulting in an increase of its pathogenicity [16].
Although it has been reported that pathogens could increase the ROS level of the host by the mechanism of pH regulation and secretion of ammonia, the direct effect of the environmental pH during the inoculation of T. roseum on the host and metabolic effect on ROS production and scavenging in apple fruit have not been analyzed. In this study, ‘Fuji’ apples were inoculated with T. roseum spore suspension at different pH levels. The cell membrane permeability, malondialdehyde content, ROS production, scavenging enzymes activities, key enzymes activity, and substrate and product contents in the AsA-GSH cycle were analyzed to determine the effects of different levels of pH on antioxidant capacity in colonized apples. This analysis suggests that the pathogens enhance pathogenicity by modulating the environmental pH, transcriptionally activating fungal pathogenicity factors but also modulate the host metabolic response.
2. Materials and Methods
2.1. Fruit and Strain
Apples (Malus domestica Borkh. cv. Fuji) were harvested from a commercial orchard in Jingtai (37°38′ N, 105°34′ E, 1671 m altitude), Gansu Province, China. The type of soil is sandy, annual average temperature is 3.1–9.1 °C, and annual rainfall is 160–450 mm. The fruits were obtained from 15-year-old trees, planted in a 5 × 5 m plantation. The cultivation was in accordance with the local cultivation standard. The fruits were neat in appearance, of uniform size, and free from pests, disease, and mechanical injury, withTotal Soluble Solids values of 14.2 (TSS). Fruits were put into corrugated paper packing boxes after each fruit was netted. The packed fruits were transported to the Postharvest Biology and Technology Laboratory at Gansu Agricultural University on the day of harvest, stored in the cold room (4 ± 2 °C, with a relative humidity of 55%–60%), and were treated two days later.
T. roseum was isolated from the core of rotten apples and cultured on potato dextrose agar (PDA) medium.
2.2. Spore Suspension with Different pH Values
Spore suspensions were prepared according to the method of Eshel et al. [17]. Three dishes of T. roseum were cultured at 28 °C for 5 days, then 10 mL of disodium hydrogen phosphate-citric acid buffer at pH 3, pH 5, and pH 7 were added to each dish. Spores on the surface of the culture medium were gently scraped off with a sterile glass rod, filtered through four layers of sterile gauze, and the concentration of the spore suspension was counted with a hemocytometer and diluted to a concentration of 1 × 106 spores/mL with sterile distilled water.
2.3. Fruit Inoculation
The fruits were inoculated according to the method of Gong et al. [18]. Apples were rewarmed for 24 h after removal from the cold room. After washing with tap water, they were surface-sterilized in 1% NaClO3 for 3 min. Then the apples were dried at room temperature, and four inoculation holes of 3 mm depth and 3 mm diameter were punched in the equator of the fruit with a sterilized iron nail. Ten microliters of spore suspensions of different pH were placed into the holes. After being dried, the fruits were placed into polyethylene bags and stored at room temperature (22 ± 2 °C, RH 85%–90%).
2.4. Maintenance of pH Value in Inoculation Site
A sterile disodium hydrogen phosphate-citrate buffer solution at pH 3, pH 5, and pH 7 was injected into the inoculation wells at 12 h intervals from the beginning of inoculation until the end of sampling time [17].
2.5. Sampling
The samples were collected according to the method described by Wang et al. [7]. At different days after inoculation, the peel and pulp of the diseased and healthy tissue (approximately 3 mm) at the leading edge junction were sampled, within 3 mm of the disease-health junction were taken, immediately frozen by liquid nitrogen, then stored at −80 °C until analyzed.
2.6. Determination of Cell Membrane Permeability and Malondialdehyde (MDA) Content
The cell membrane permeability was determined according to the method of Lester et al. [19] with minor modifications. Ten grams of samples were incubated in 40 mL of deionized water, and the conductivity was determined at 0 h and 3 h of incubation with a conductivity meter (DDS-307A; RIDAO, Shanghai) at 25 °C and recorded as E0 and E1, respectively. After that the samples were incubated in boiling water for 0.5 h, and then quickly cooled to determine the conductivity which was recorded as E2. The cell membrane permeability was calculated using the following formula:
| Cell membrane permeability (%) = (E1 − E0)/ E2 × 100% |
The MDA content was determined according to the method of Hodges et al. [20] with slight modifications. Frozen tissue (3 g) was homogenized with 6 mL precooled trichloroacetic acid (TCA) extract solution, and then centrifuged for 20 min at 12 000× g, at 4 °C. The absorbance of the reaction of 2 mL supernatant with 2 mL 0.67% (w/v) 2-thiobarbituric acid (TBA) was determined at 450 nm, 532 nm, and 600 nm after incubating in boiling water for 20 min. The MDA content was calculated according to the formula and expressed as nmol/g FW.
| CMDA (μmol/L) = 6.45 × (OD532 − OD600) − 0.56 × OD450 |
| MDA content (nmol/g FW) = CMDA (μmol/L) × Extraction volume (mL)/Fruit fresh weight (g) |
2.7. Determination of NADPH Oxidase and Superoxide Dismutase (SOD) Activity
The NADPH oxidase activity was determined according to the method of Morré et al. [21] with slight modifications. Frozen tissue (3 g) was homogenized with 5 mL extract solution, and centrifuged at 4 °C and 7500× g for 15 min. The reaction mixture for NADPH oxidase activity contained 2 mL 50 mM Tris-HCl buffer (pH 7.5), 0.5 mM sodium 3,3′-[1-(Benzyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulfonate (XTT), 100 µM NADPH, and 200 µL microcapsule of cell membrane. The activity of NADPH oxidase was expressed as U g−1 FW, where U was defined as an increasing of 0.01 in absorbance per minute at 470 nm.
The SOD activity was determined according to the method of Supapvanich et al. [22] with slight modifications. Frozen tissue (3 g) was homogenized with 5 mL 50 mM phosphate buffer (pH 7.8). The reaction system contained 1.7 mL phosphate buffered saline (PBS) solution (pH 7.8, 50 mM), 300 μL 130 mM methionine, 300 μL 750 μM nitro blue tetrazolium (NBT), 0.3 mL 100 μM EDTA-Na2, 0.1 mL crude enzyme solution, and 100 μL riboflavin. The absorbance was measured at 560 nm. 50% inhibition of photoreduction of NBT and expressed as U g−1 FW.
2.8. Determination of Rate of O2●− Production and H2O2 Content
The rate of O2●− production was determined according to the method of Luo et al. [23] with some modifications. Frozen tissue (3 g) was homogenized with 5 mL 100 mM phosphate buffer (0.1% PVPP, pH 7.8) and centrifuged at 4 °C and 12,000× g for 20 min. Then 1 mL of supernatant was mixed with 1 mL 50 mM phosphate buffer (pH 7.8) and 1 mL 1 mM hydroxylamine hydrochloride, and incubated for 1 h at 25°C. Then 1 mL 17 mM p-aminobenzenesulfonic acid and 1 mL 7 mM α-naphthylamine were added and incubated at 25 °C for 20 min. The absorbance was measured at 530 nm. The O2●− production rate was expressed as nmol/min/g FW.
The H2O2 content was determined using a kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China). Frozen tissue (3 g) was homogenized with 5 mL 1 mM phosphate buffer (pH 7). The reagents were added according to the instructions of the kit for H2O2 content determination. Absorbance was measured at 405 nm. The content of H2O2 was expressed as μmol g−1 FW.
2.9. Determination of Catalase (CAT) and Peroxidases (POD) Activities
The CAT activity was measured according to Fan et al. [24]. Frozen tissue (3 g) was homogenized with 5 mL 0.1 M sodium phosphate buffer. The homogenate was then centrifuged at 4 °C and 12,000× g for 20 min. The reaction mixture for CAT activity contained 50 mM phosphate buffer (pH 7.5), 20 mM H2O2, and 100 μL of crude enzyme. The activity of CAT was expressed as U g−1 FW, where U was defined as an increase of 0.01 in absorbance per minute at 240 nm.
POD activity was measured according to Venisse et al. [25]. Frozen tissue (3 g) was homogenized with 5 mL of 0.1 M acetic acid-sodium acetate buffer (containing 1% TritonX-100, 4% PVPP, and 1 mM PEG, pH 5.5). The homogenate was then centrifuged at 4 °C and 12,000× g for 20 min. The reaction mixture for POD contained 0.5 mL of 25 mM guaiacol, 200 μL of 30% H2O2, and 500 μL of crude enzyme extract. The activity of POD was expressed as U g−1 FW, where U was defined as an increase of 0.01 in absorbance per minute at 470 nm.
2.10. Determination of Key Enzyme Activities of the AsA-GSH Cycle
Crude enzyme solution was extracted according to the method described by Nakano and Asada [26]. Frozen tissue (3 g) was homogenized with 3 mL of pH 7.5 100 mM phosphate buffer (1 mM EDTA), and then centrifuged at 4 °C and 12 000× g for 30 min.
Ascorbate peroxidase activity was measured according to the method reported by Nakano and Asada [26]. The reaction system contained 2 mL 100 mM phosphate buffer (pH 7.5, containing 1 mM EDTA), 0.8 mL 3 mM ascorbic acid, 200 μL crude enzyme solution, and 0.5 mL 0.5 mM H2O2. The absorbance of the reaction system at 290 nm was recorded from 15 s after start-up for 2 min. The activity of APX was expressed as U g−1 FW, where U was defined as an increase of 0.01 in absorbance per minute at 290 nm.
Monodehydroascorbate reductase activity was measured according to the method reported by Nakano and Asada [26]. The reaction system was 2 mL 40 mM phosphate buffer solution (pH 8.0), 0.2 mL 10 mM sodium ascorbate, 0.1 mL 40 μM copper sulphate solution, 0.5 mL crude enzyme solution, and 0.2 mL of 0.2 mM NADPH. The absorbance at 340 nm was measured immediately after mixing and recorded for 2 min. The activity of DHAR was expressed as U g−1 FW, where U was defined as an increase of 0.01 in absorbance per minute at 340 nm.
Dehydroascorbate reductase activity was measured according to the method reported by Nakano and Asada. [26]. The reaction system was 100 μL crude enzyme solution, 300 μL 0.1 mM EDTA-Na2, 400 μL 2 mM GSH, 400 μL 0.5 mM DHA, and 2 mL 40 mM phosphate buffer solution (pH 8.0). The absorbance at 290 nm was measured immediately after mixing. Changes of reaction system within 2 min were recorded. The activity of DHAR was expressed as U g−1 FW, where U was defined as an increase of 0.01 in absorbance per minute at 290 nm.
Glutathione reductase activity was measured according to the method reported by Halliwell et al. [27]. The reaction system was 3 mL 100 mM phosphate buffer, 0.1 mL 5 mM oxidized glutathione (GSSG), 30 μL 3 mM NADPH, and 0.2 mL crude enzyme solution. NADPH was added to initiate the enzymatic reaction. The absorbance was measured at 340 nm for 2 min. The activity of GR was expressed as U g−1 FW, where U was defined as an increase of 0.01 in absorbance per minute at 340 nm.
2.11. Determination of AsA-GSH Cycle Substrates and Products Contents
Ascorbic acid and dehydroascorbate content were determined using a kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). AsA was oxidized by ascorbatease AAO to generate DHA, and the AsA content was calculated by measuring the oxidation rate of AsA. AsA content was expressed as nmol/g FW. DHA can be reduced to AsA by DTT, so DHA content can be calculated by measuring AsA formation rate in the system. DHA content was expressed as nmoL/g FW. Determination of GSSG and GSH content was conducted using a kit (Suzhou Keming Biotechnology Co., Ltd.). DTNB reacts with GSH to form a complex with characteristic absorption peak at 412 nm, the absorbance is proportional to GSH content. GSSG and GSH of content were expressed as nmol/g FW.
2.12. Statistical Analysis
Data were expressed as means ± standard errors. Figures were prepared with software Origin 8.0 (Northampton, MA, USA). Differences between treatments were analyzed using an ANOVA, followed by LSD multiple range tests at a 0.05 significance level using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Effect of Inoculation of T. roseum at Different pH Conditions on Cell Membrane Permeability and MDA Content of Colonized Apple Tissue
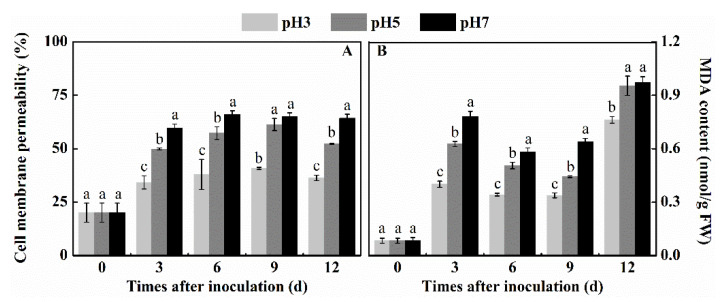
Cell membrane permeability and MDA content are important indexes reflecting the integrity of the cell membranes of the colonized tissue. Cell membrane permeability of fruits inoculated with spore suspensions at different pH levels, showed increasing cell permeability at increasing pH levels. Cell membrane permeability of pH 7 treatment showed the highest activity compared to pH 5 and pH 3 by 23.2% and 77% by the 12th day, respectively (p < 0.05) (Figure 1A). In addition, the content of MDA increased as the environmental pH values increased from pH 3 to pH 7. Environmental pH of 7 led to a significantly higher accumulation of MDA than treatments with pH 5 and pH 3 during the first 9 days after inoculation. By the 9th day after inoculation, MDA levels at pH 7 showed values 44% and 89% higher than the values found at pH 5 and pH 3, respectively (p < 0.05) (Figure 1B). These results suggest that at increasing environmental pH conditions of colonization by T. roseum, the fungus induces enhanced host permeability and MDA accumulation that may contribute to the enhanced colonization at higher pH values.
Figure 1.
Effect of inoculation of T. roseum at different pH conditions on cell membrane permeability (A) and malondialdehyde content (B) of colonized apple fruits. Bars indicate standard error (±SE). Different letters indicate statistically significant differences (p < 0.05) among treatments.
3.2. Effect of Inoculation of T. roseum at Different pH Conditions on NADPH Oxidase and SOD Activities of Colonized Apple Tissue
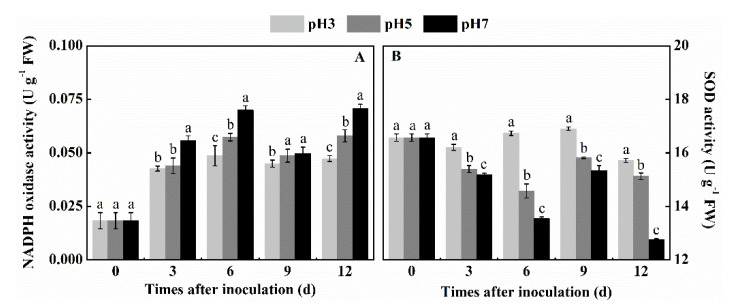
NADPH oxidase and SOD are two important enzymes which produce O2●− and H2O2, respectively. Colonized fruits at increasing environmental pH conditions showed increasing NADPH oxidase activities. NADPH oxidase activity showed a positive regulation with the increasing of pH. At the environmental pH 7 it showed 23.8% and 49.3% higher levels than at pH 5 and pH 3 by the 12th day after inoculation, respectively (p < 0.05) (Figure 2A). In contrast to NADPH Oxidases, the SOD activity showed a negative regulation of the activity with the increasing of environmental pH. While the SOD activity at pH 3 was not affected in the days after inoculation; exposure of the pathogen to the environmental pH 7 showed a continuous decrease with the increasing days after inoculation (p < 0.05) (Figure 2B). The above results indicated that environmental pH increasing at the wound inoculation spot enhanced NADPH oxidase but inhibited SOD activity in apple fruits.
Figure 2.
Effect of inoculation of T. roseum at different pH conditions on the activities of NADPH oxidase (A) and SOD (B) of colonized apple fruits. Bars indicate standard error (±SE). Different letters indicate statistically significant differences (p < 0.05) among treatments.
3.3. Effect of Inoculation of T. roseum at Different pH Spore Suspensions on the Rate of O2●− Production and H2O2 of Colonized Apple Tissue
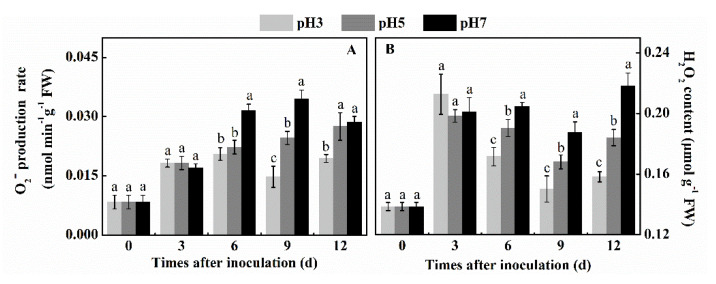
O2●− production rate and H2O2 content directly reflect the accumulation level of ROS. O2●− production rate of fruits inoculated with spores at three pH suspensions showed an overall increase. At pH 7 there was an overall higher O2●− production than pH 5 and pH 3. At the 9th day after inoculation, the increase in O2●− production rate was 40% and 30% higher than that of pH 5 and pH 3, respectively (p < 0.05) (Figure 3A). The H2O2 accumulation showed a different pattern. While the H2O2 content of pH 7 treatment increased within 3 days as a result of the inoculation with the pH 7 treatment, it maintained the level of H2O2 production during all the 12 days of the evaluation. In contrast, the inoculation of spores and environmental pH 3 showed only a single peak of response, 3 days after inoculation followed by a significant decline response up to day 9–12 after inoculation suggesting that there is a different pattern of response between pH 3 and pH 7. Inoculation at pH 5 also showed a similar decline in response to the treatment as the pH 3, however, with an intermediate response between the pH 3 and pH 7 treatment (p < 0.05) (Figure 3B). The above results showed that inoculation at increasing pH increased the O2●− production rate. However, the pH effect on the H2O2 content was more complex, showing different responses between pH 3 and pH 7.
Figure 3.
Effect of inoculation of T. roseum at different pH conditions on the O2●− production rate (A), and H2O2 content (B) of colonized apple fruits. Bars indicate standard error (±SE). Different letters indicate statistically significant differences (p < 0.05) among treatments.
3.4. Effect of Inoculation of T. roseum at Different pH Conditions on the Activities of CAT and POD of Colonized Apple Tissue
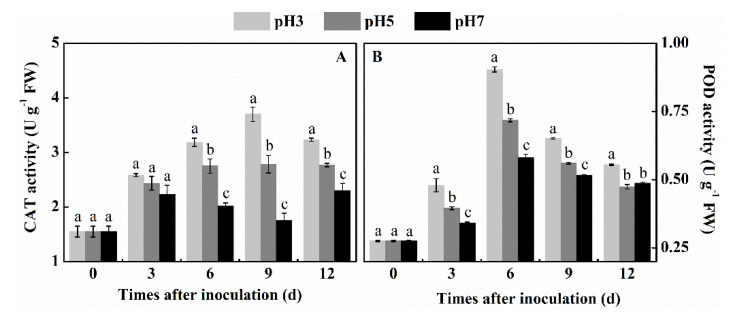
CAT and POD are the two key enzymes for H2O2 scavenging. CAT activity of fruit inoculated with three pH spore suspensions showed an overall increase in activity, but the trend was different between pH 3 and pH 7. With the pH 3 treatment there was a 2-fold increase in enzyme activity by the 3rd day after inoculation, and a 4-fold increase by the 9th and 12th day after inoculation. The fruit response to the inoculation at pH 7 was significantly minor, showing by the 9th day almost no difference of response with the untreated control tissue. At pH 5 an intermediate response with a similar pattern of activity as at pH 3 was observed, suggesting that as the pH increased the level of response of CAT significantly declined (p < 0.05) (Figure 4A). POD, an antioxidant that is an endogenous ROS scavenger, again showed the highest increase when spores were inoculated at pH 3 with a 3-fold increase by the 6th day after inoculation and a minimal response when spores were inoculated at pH 7. Interestingly, no response at all was observed by the 3rd day of inoculation, with a slight increase on days 6, 9, and 12 after inoculation (p < 0.05) (Figure 4B). The above results indicate a higher level of antioxidant response at lower pH levels and may suggest the importance of the pH increase during fruit colonization.
Figure 4.
Effect of inoculation of T. roseum at different pH conditions on the activity of CAT (A) and POD (B) of colonized apple fruits. Bars indicate standard error (±SE). Different letters indicate statistically significant differences (p < 0.05) among treatments.
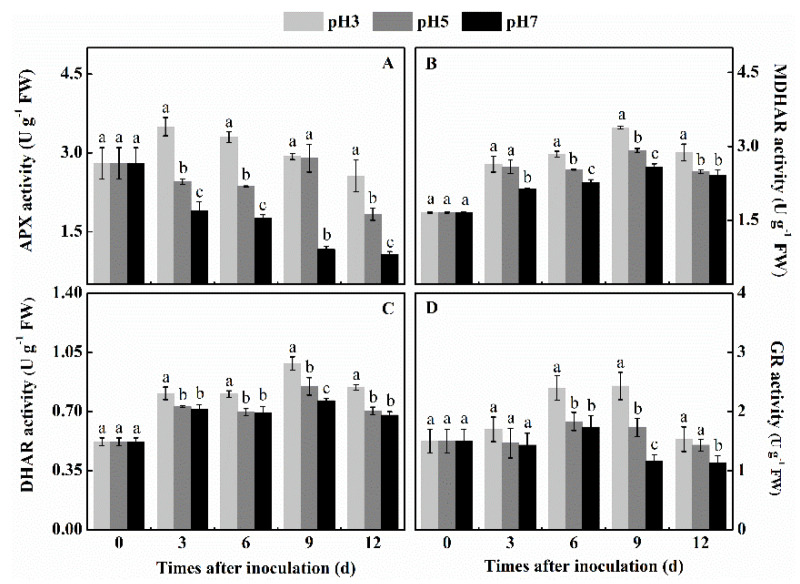
3.5. Effect of Inoculation of T. roseum at Different pH Conditions on the Activities of APX, MDHAR, DHAR, and GR of Colonized Apple Tissue
APX, MDHAR, DHAR, and GR are involved in the AsA-GSH cycle and play an important role in maintaining AsA and GSH levels as regulators of the redox balance in plants. APX, MDHAR, DHAR, and GR are also H2O2-scavenging enzymes and are indispensable for the protection of chloroplasts and other cell constituents from damage by H2O2 in fruits. The inoculation of spores at pH 3 in all the 4 enzymes analyzed showed a significantly increased response during the 12 days of evaluation. For MDHAR and DHAR at pH 7 the level of increase of activity was 20%–30% lower than for pH 3 (p < 0.05) (Figure 5B,C). However, for APX and GR activities, the level of activities were even inhibited compared to those at pH 3 (Figure 5A,D). This clearly indicates that while the antioxidant activity pattern at pH 3 is induced, the response at pH 7 is significantly inhibited or not activated.
Figure 5.
Effect of inoculation of T. roseum at different pH conditions on the activity of APX (A), MDHAR (B), DHAR (C), and GR (D) of colonized apple fruits. Bars indicate standard error (±SE). Different letters indicate statistically significant differences (p < 0.05) among treatments.
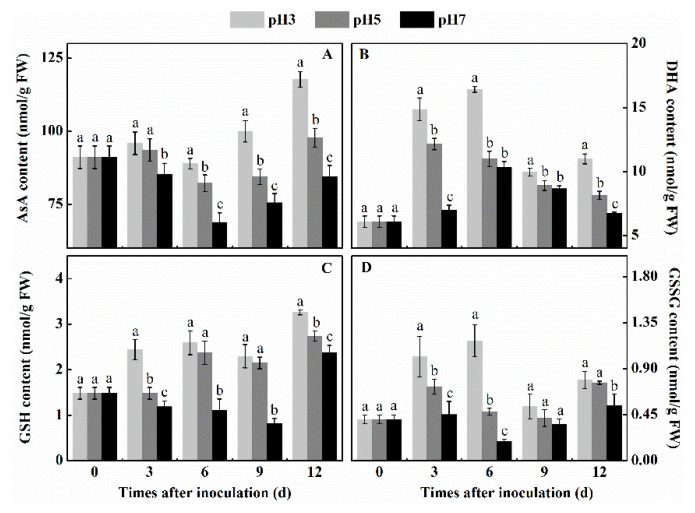
3.6. Effect of Inoculation of T. roseum at Different pH Conditions on the Contents of AsA, DHA, GSH, and GSSG of Colonized Apple Tissue
AsA and GSH are the main antioxidant substances in the ASA-GSH cycle, which can scavenge H2O2 through the ASA-GSH cycle. The general pattern of response of the 4 antioxidants showed a negative response as the pH increased (Figure 6). While there was a differential increase in the level of antioxidants, there was a clear and significant decrease at pH 7 compared to the colonization at pH 3. Analysis of each of the antioxidants showed two different responses: two of these antioxidants ASA and GSH (Figure 6A,C) showed patterns of continuous (in general) increase in concentrations when the inoculation was carried out at pH 3 at different periods after inoculation, but the other two, GSH and GSSG (Figure 6B,D) showed only an initial positive response after inoculation (3 and 6 days) and then a decline in the concentrations (9 and 12 days) at similar time levels as at pH 7. These data indicate that the activation of the antioxidant content is strongly dependent on the pH, being higher at low pH values, but still showing differential levels of accumulation according to the specific antioxidant.
Figure 6.
Effect of inoculation of T. roseum at different pH conditions on the activity of AsA (A), DHA (B), GSH (C), and GSSG (D) of colonized apple fruits. Bars indicate standard error (±SE). Different letters indicate statistically significant differences (p < 0.05) among treatments.
4. Discussion
Postharvest pathogens have the ability to alkalinize the environment, contributing to fungal pathogenesis by activation of genes that modulate the pathogenicity factors [28]. Inoculation of apple fruits with spore suspensions of T. roseum at increasing pH values, from pH 3 to pH 7, enhanced a faster colonization of the apple tissue and the enhancement of secretion of pectolytic enzymes, as a result of the enhanced pH induced by the pathogen [7]. However, the increase of pH modulated host physiological responses in the apple tissue. Inoculation of spores of the pathogen at pH 7 induced a significant increase in NADPH oxidase activity, a faster rate of increase of O2 production, the highest H2O2 accumulation, and reduction of cell membrane integrity. A similar pattern of responses was observed for the secretion of ammonia by C. coccodes, which activated the tomato fruit RBOH activity, thus regulating the host H2O2 level [16]. Given that ammonia can activate H+-ATPase activity, which affects the influx of Ca2+ [14], it is possible that Ca2+ can activate calcium-dependent protein kinases (CDPKs) at downstream targets [29] and then generate of O2●− by phosphorylating NADPH oxidase [30]. Therefore, fungal alkalization of the environment might activate the cell membrane proton pump H+-ATPase under the higher pH 7 environment, leading to Ca2+ influx, which could further activate CDPK, resulting in an accumulation of ROS. The ROS could directly attack the membrane system, thus destroying the integrity of the cell membrane, and contribute to the enhanced fungal colonization. However, this still requires further study to fully elucidate this mechanism.
ROS is an important virulence factor of pathogens, which can react with lipids, proteins, nucleic acids, and other biological macromolecules of host cells, leading to lipid peroxidation, protein cross-linking, DNA damage, and so forth, finally resulting in the programmed death of host cells [31]. However, other antioxidant systems regulate the accumulation of ROS in the fruit tissue. The present observations indicated that the SOD, CAT, and POD activities were strongest in pH 3 inoculated fruits and weakest in pH 7 inoculated fruits. These activities are important given that SOD is the only enzyme that can interact with O2●− to generate H2O2 in organisms [32]. CAT is also one of the key enzymes in the antioxidant system of plants, and its function is to scavenge H2O2, which can reduce H2O2 to H2O and O2 [33] showing a closely related activity to the redox balance in plant cells [34]. POD is considered an endogenous ROS scavenger in plant cells, working in concert with CAT to form the antioxidant enzyme system of plants [35,36]. Previous reports indicated that transcript analysis of SOD, CAT, and POD in petunia showed significantly higher activities at ambient pH 3 than pH 5.8 [37]. Similarly, higher activities of both SOD and CAT were observed at pH 3 than pH 7 in Rosa hybrida (cv. Tereasa) [38], supporting the present study. Therefore, it is clear that lower pH values were more conducive to maintaining the activity of SOD, CAT, and POD than higher pH environments. However, another antioxidative system was also significantly activated at lower pH. The AsA-GSH cycle is an important H2O2 scavenging system in plants, which plays an indispensable role in the effective removal of H2O2 [39]. Our results indicate that in apples colonized by T. roseum APX, MDHAR, DHAR, and GR activities, as well as the levels of AsA, DHA, GSH, and GSSG at pH 3, were significantly higher than at pH 5 and pH 7. APX is the first enzyme in the cycle that specifically catalyzes the reaction of AsA with H2O2 to form MDHA [40]. Results obtained in colonized tissue with Trichotecium were similar to those found in ginger seedlings that showed strong activity of the AsA-GSH cycle enzymes under low pH conditions [12]. Yin et al. found that low pH improved APX activity and increased glutathione and ascorbic acid content, thus effectively scavenging H2O2 and protecting the integrity of membrane lipids [12]. In fruit systems such as kiwi fruit treated with 5 mM oxalic acid (pH 3.1), the low pH increased the AsA content, thereby enhancing the ROS scavenging ability and reducing the damage of membrane lipid peroxidation [41]. Therefore, we speculated that low pH conditions could increase the activity of the AsA-GSH cycle, and thus effectively scavenge excessive H2O2. In summary, we hypothesize that the colonization by T. roseum of apple fruit, under reduced pH conditions, induced the activation of the AsA-GSH cycle, contributing to the reduced colonization of the fungus in apple fruit.
5. Conclusions
Apple fruits colonized by T. roseum spore suspensions at different pH values modulated the activity of pectolytic and cellulolytic enzymes at increasing pH values, but the local environmental pH levels have differential effects on ROS production, cell membrane integrity, and ROS scavenging systems. Fruits colonized at pH 3 showed the lowest rate of O2●− production and H2O2 accumulation, the highest cell membrane integrity, and SOD, CAT, and POD activities, and the strongest AsA-GSH cycle activity. However, the metabolic responses at the highest pH tested were the opposite. These results suggest that the metabolic response of the fruit resulting from the alkalization by the secretion of ammonia is a key factor for modulation of the colonization of the fruit by Trichotecium. The present results indicate that fungal pH modulation of the environment may modulate fungal metabolic processes as well as fruit metabolic processes, both of them contributing to the modulation of fungal colonization by the pathogen in ripening fruits [42].
Author Contributions
Data curation, methodology, writing—review and editing, and writing—original draft, Z.H. and Z.W.; funding acquisition, resources, validation, Y.B.; supervision, visualization, Y.Z.; software, D.G.; investigation, B.W.; formal analysis, B.L.; conceptualization, D.P. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (31861143046).
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to "not applicable” for studies not involving human or animals.
Informed Consent Statement
Non applicable for studies not involving human.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Denison S.H. pH regulation of gene expression in fungi. Fungal Genet. Biol. 2000;29:61–71. doi: 10.1006/fgbi.2000.1188. [DOI] [PubMed] [Google Scholar]
- 2.Prusky D., Yakoby N. Pathogenic fungi: Leading or led by ambient pH. Mol. Plant Pathol. 2003;4:509–516. doi: 10.1046/j.1364-3703.2003.00196.x. [DOI] [PubMed] [Google Scholar]
- 3.Manteau S., Abouna S., Lambert B., Legendre L. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiol. Ecol. 2003;43:359–366. doi: 10.1111/j.1574-6941.2003.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 4.Bateman D.F., Beer S.V. Simultaneous production and syner-gistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotium rolfsii. Phytopathology. 1965;58:204–211. [PubMed] [Google Scholar]
- 5.Ruijter G.J.G., van de Vondervoort P.J.I., Visser J. Oxalic acid production by Aspergillus niger: An oxalate-non-producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology. 1999;145:2569–2576. doi: 10.1099/00221287-145-9-2569. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y. Post-harvest Diseases of Fruits and Vegetables, Symptoms and Pathogens. In: Bi Y., editor. Postharvest Diseases of Fruits and Vegetables: Principle and Control. Science Press Publishing; Beijing, China: 2016. pp. 5–6. [Google Scholar]
- 7.Wang Z.Y., Hu H.M., Gong D., Zhang G.J., Prusky D., Bi Y. Acid-base property of Trichothecium roseum and effect of pH on its extracellular enzyme activities and pathogenicity. Food Sci. 2019;40:161–166. (In Chinese) [Google Scholar]
- 8.Zhang Z.Q., Chen Y., Li B.Q., Chen T., Tian S.P. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. Comput. Struct. Biotechnol. J. 2020;4:3344–3349. doi: 10.1016/j.csbj.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao G.H., Bi Y., Li Y.C., Kou Z.H., Hu L.G., Ge Y.H., Wang Y., Wang D. Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers. Physiol. Mol. Plant Pathol. 2014;86:35–42. doi: 10.1016/j.pmpp.2014.01.004. [DOI] [Google Scholar]
- 10.Kuźniak E., Skłodowska M. Compartment-specific role of the ascorbate-glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. J. Exp. Bot. 2005;56:921–933. doi: 10.1093/jxb/eri086. [DOI] [PubMed] [Google Scholar]
- 11.Bolwell G.P. The origin of the oxidative burst in plants. Biochem. Soc. Trans. 1996;24:438–442. doi: 10.1042/bst0240438. [DOI] [PubMed] [Google Scholar]
- 12.Yin F., Liu X., Cao B., Xu K. Low pH altered salt stress in antioxidant metabolism and nitrogen assimilation in ginger (Zingiber officinale) seedlings. Physiol. Plant. 2020;168:648–659. doi: 10.1111/ppl.13011. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Mao L.C., Li X.W., Lv Z., Liu C.H., Huang Y.Y., Li D.D. Oxalic acid pretreatment reduces chilling injury in Hami melons (Cucumis melo var. reticulatus Naud.) by regulating enzymes involved in antioxidative pathways. Sci. Hortic. 2018;241:201–208. doi: 10.1016/j.scienta.2018.06.084. [DOI] [Google Scholar]
- 14.Bai X.D., Bi Y., Li Y.C., Wang Y., Niu L.L., Wang T., Shang Q. Mechanism of latent infection for postharvest diseases of fruits and vegetables. Food Sci. 2015;36:298–302. [Google Scholar]
- 15.Zhang R., Xue H.L., Si M., Bi Y., Nan M.N., Zong Y.Y., Long H.T., Prusky D., Cheng X.Y. Mechanism of Ca2+-mediated NOX modulated in ROS metabolism induced by T-2 toxin in potato tuber. Food Chem. 2020;317:126416. doi: 10.1016/j.foodchem.2020.126416. [DOI] [PubMed] [Google Scholar]
- 16.Alkan N., Davydov O., Sagi M., Fluhr R., Prusky D. Ammonium secretion by Colletotrichum coccodes activates host NADPH oxidase activity enhancing host cell death and fungal virulence in tomato fruits. Mol. Plant Microbe Interact. 2009;22:1484–1491. doi: 10.1094/MPMI-22-12-1484. [DOI] [PubMed] [Google Scholar]
- 17.Eshel D., Miyara I., Ailing T., Dinoor A., Prusky D. pH regulates endoglucanase expression and virulence of Alternaria alternata in persimmon fruit. Mol. Plant Microbe Interact. 2002;15:774–779. doi: 10.1094/MPMI.2002.15.8.774. [DOI] [PubMed] [Google Scholar]
- 18.Gong D., Bi Y., Jiang H., Xue S.L., Wang Z.Y., Li Y.C., Zong Y.Y., Prusky D. A comparison of postharvest physiology, quality and volatile compounds of ‘Fuji’ and ‘Delicious’ apples inoculated with Penicillium expansum. Postharvest Biol. Technol. 2019;15:95–104. doi: 10.1016/j.postharvbio.2018.12.018. [DOI] [Google Scholar]
- 19.Lester G.E., Bruton B.D. Relationship of netted muskmelon fruit water loss to postharvest storage life. J. Am. Soc. Hortic. Sci. 1986;111:727–731. [Google Scholar]
- 20.Hodges D.M., Delong J.M., Prange F.R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- 21.Morré D.J., Morré D.M. Applications of aqueous two-phase partition to isolation of membranes from plants: A periodic NADH oxidase activity as a marker for right side-out plasma membrane vesicles. J. Chromatogr. B Biomed. Sci. Appl. 2000;743:369–376. doi: 10.1016/S0378-4347(00)00033-5. [DOI] [PubMed] [Google Scholar]
- 22.Supapvanich S., Mitsang P. Preharvest salicylic acid application maintains physicochemical quality of ‘Taaptimjaan’ wax apple fruit (Syzygium samarangenese) during short-term storage. Sci. Hortic. 2017;215:178–183. doi: 10.1016/j.scienta.2016.11.046. [DOI] [Google Scholar]
- 23.Luo G.H., Wang A.G., Guo J.Y. Effect of several exogenous factors on the SOD activity of soybean seedlings. Sci. Hortic. 1990;3:239–244. (In Chinese) [Google Scholar]
- 24.Fan M.C., Li W.X., Hu X.L. Effect of micro-vacuum storage on active oxygen metabolism, internal browning and related enzyme activities in Laiyang pear (Pyrus bretschneideri Reld) LWT Food Sci. Technol. 2016;72:467–474. doi: 10.1016/j.lwt.2016.05.015. [DOI] [Google Scholar]
- 25.Venisse J.S., Gullner G., Brisset M.N. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol. 2001;125:2164–2172. doi: 10.1104/pp.125.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 27.Halliwell B., Foyer C.H. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta. 1978;139:9–17. doi: 10.1007/BF00390803. [DOI] [PubMed] [Google Scholar]
- 28.Prusky D., McEvoy J.L., Leverentz B., Conway W.S. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant Microbe Interact. 2001;14:1105–1113. doi: 10.1094/MPMI.2001.14.9.1105. [DOI] [PubMed] [Google Scholar]
- 29.Nürnberger T., Scheel D. Signal transmission in the plant immune response. Trends Plant Sci. 2001;6:372–379. doi: 10.1016/S1360-1385(01)02019-2. [DOI] [PubMed] [Google Scholar]
- 30.Adachi H., Yoshioka H. Kinase-mediated orchestration of NADPH oxidase in plant immunity. Brief. Funct. Genom. 2015;14:253–259. doi: 10.1093/bfgp/elv004. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Ji D.C., Chen T., Li B.Q., Zhang Z.Q., Qin G.Z., Tian S.P. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit-pathogen interactions. Int. J. Mol. Sci. 2019;20:2994. doi: 10.3390/ijms20122994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Y.H., Chen Y.R., Li C.Y., Zhao J.R., Wei M.L., Li X.H., Yang S.Q., Mi Y.T. Effect of sodium nitroprusside treatment on shikimate and phenylpropanoid pathways of apple fruit. Food Chem. 2019;290:263–269. doi: 10.1016/j.foodchem.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Krantev A., Yordanova R., Janda T., Szalai G., Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008;165:920–931. doi: 10.1016/j.jplph.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Li Q., Wang G., Wang Y.R., Yang D., Guan C.F., Ji J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Environ. Saf. 2019;172:317–325. doi: 10.1016/j.ecoenv.2019.01.078. [DOI] [PubMed] [Google Scholar]
- 35.Ren Y.L., Wang Y.F., Bi Y. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Eur. Food Res. Technol. 2012;234:963–971. doi: 10.1007/s00217-012-1715-x. [DOI] [Google Scholar]
- 36.Zeng K.F., Deng Y.Y., Ming J.A., Deng L.L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010;126:223–228. doi: 10.1016/j.scienta.2010.07.017. [DOI] [Google Scholar]
- 37.Naing A.H., Lee D.B., Ai T.N., Lim K.B., Kim C.K. Enhancement of low pH stress tolerance in anthocyanin-enriched transgenic petunia overexpressing RsMYB1 gene. Front. Plant Sci. 2018;9:1124. doi: 10.3389/fpls.2018.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shanan N. Optimum pH value for improving postharvest characteristics and extending vase life of Rosa hybrida cv. Tereasa cut flowers. Asian J. Adv. Agric. Res. 2017;1:1–11. doi: 10.9734/AJAAR/2017/34655. [DOI] [Google Scholar]
- 39.Shan C.J., Wang B.S., Sun H.L., Gao S., Li H. H2S induces NO in the regulation of AsA-GSH cycle in wheat seedlings by water stress. Protoplasma. 2020;257:1487–1493. doi: 10.1007/s00709-020-01510-3. [DOI] [PubMed] [Google Scholar]
- 40.Imahori Y., Bai J., Baldwin E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016;198:398–406. doi: 10.1016/j.scienta.2015.12.006. [DOI] [Google Scholar]
- 41.Ding Z.S., Tian S.P., Zheng X.L., Zhou Z.W., Xu Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol. Plant. 2007;130:112–121. doi: 10.1111/j.1399-3054.2007.00893.x. [DOI] [Google Scholar]
- 42.Bi F., Barad S., Ment D., Luria N., Casado V., Galam N., Dubay A., Mínguez J.D., Espeso E., Fluhr R., et al. Carbon regulation of environmental pH by secreted small molecule effectors modulates pathogenicity in fungi. Mol. Plant Pathol. 2016;17:1178–1195. doi: 10.1111/mpp.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository.