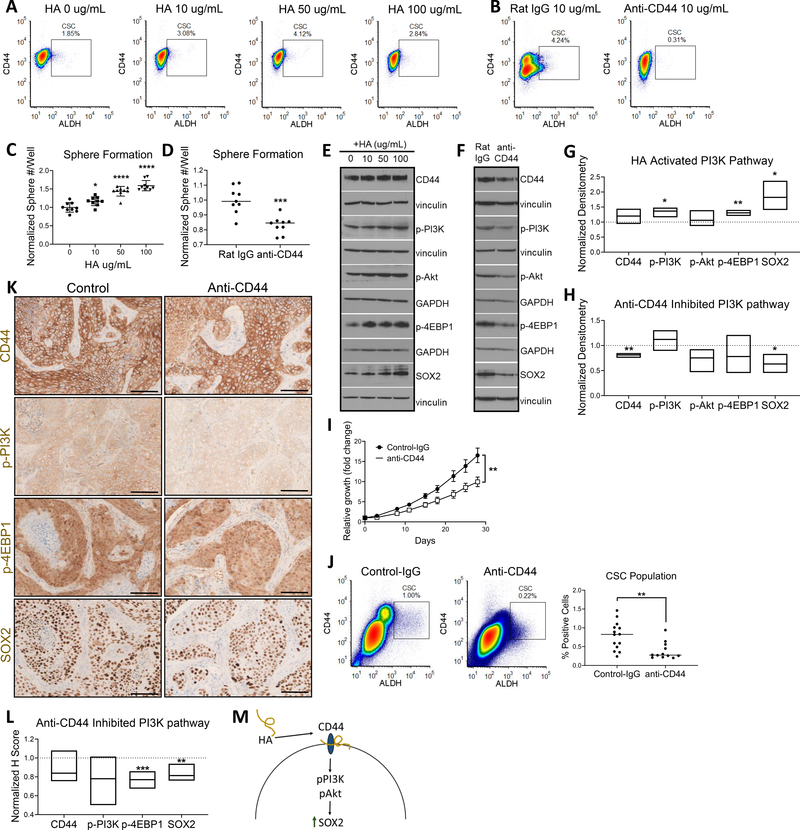
Figure 3. HA-CD44 promotes stemness via PI3K-4EBP1-SOX2 pathway activation.
(A) HA treatment increased the percent CSC population as measured via flow cytometry. (B) HA binding blockade with anti-CD44 mAb decreased percent CSC population. (C) Cancer cell sphere formation increased with increasing doses of HA. (D) Cancer cell sphere formation decreased with ligand blockade. (E) WB of activated PI3K-4EBP1-SOX2 pathway with HA treatment. (F) WB of lowered PI3K-4EBP1-SOX2 pathway activation with anti-CD44 mAb treatment. For E and F, vinculin and GAPDH were used as loading controls. (G) Floating bar plot of normalized WB densitometry of 3 repeats for 50μg/mL HA. Line at y = 1 represents protein expression of 0μg/mL HA control. (H) Floating bar plot of normalized WB densitometry of 3 repeats. Line at y = 1 represents protein expression of treatment with Rat IgG isotype control. (I) In vivo treatment of NSG mice with anti-CD44 mAb results in reduced tumor growth. Tumor growth curve (top) and final tumor size comparison (bottom) (IgG = 14 tumors, anti-CD44 = 12 tumors). (J) In vivo treatment of NSG mice with anti-CD44 mAb results in decreased percent CSC population. (K) In vivo treatment with anti-CD44 mAb decreases CD44-pPI3K-p4EBP1-SOX2 tumor expression (L) Normalized H score of anti-CD44 treated tumor IHC. Line at y = 1 represents average H score of control tumors (n = 6 tumors each). (M) Mechanistic overview of promotion of PI3K-4EBP1-SOX2 pathway signaling due to CD44 ligand binding. For C and D, 3 wells plated per repeat and 3 repeats performed. Statistical significance in C, D, G, H, I and J determined by 2-tailed unpaired Student’s t test *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Scale bars: 100μm.