Figure 1.
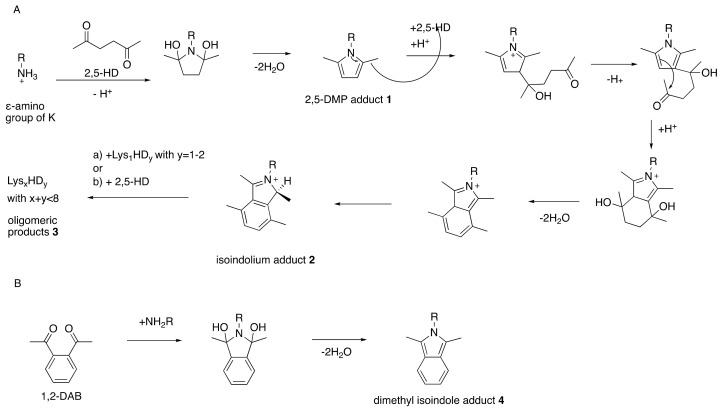
Interconvertibility of aliphatic and aromatic γ-diketones and their respective reactions with amino groups to form pyrrole (A) and isoindolium (B) adducts, respectively. Structures 1, 2, and 4 are experimentally observed and 5–8 are hypothesized [37]. Rats treated with 1,2-diacetylbenzene (1,2-DAB) develop a bluish coloration whereas 2,5-hexanedione (2,5-HD)-treated animals do not. This can be explained by the extended aromatic system in the case of 8 compared to 5 [37]. Even though 2,5-HD-derived 2 has high structural similarities with 1,2-DAB-derived 4 and 7, the reaction of 2,5-HD-induced 3 will not hypothetically proceed to 8 in vivo. From Trimpin, S.; Hsu, V.L.; Spencer, P.S.; Deinzer, M.L. Time-dependent 2,5-hexanedione adduction of lysine residues to isoindolium cation. Unpublished [38].