Figure 4:
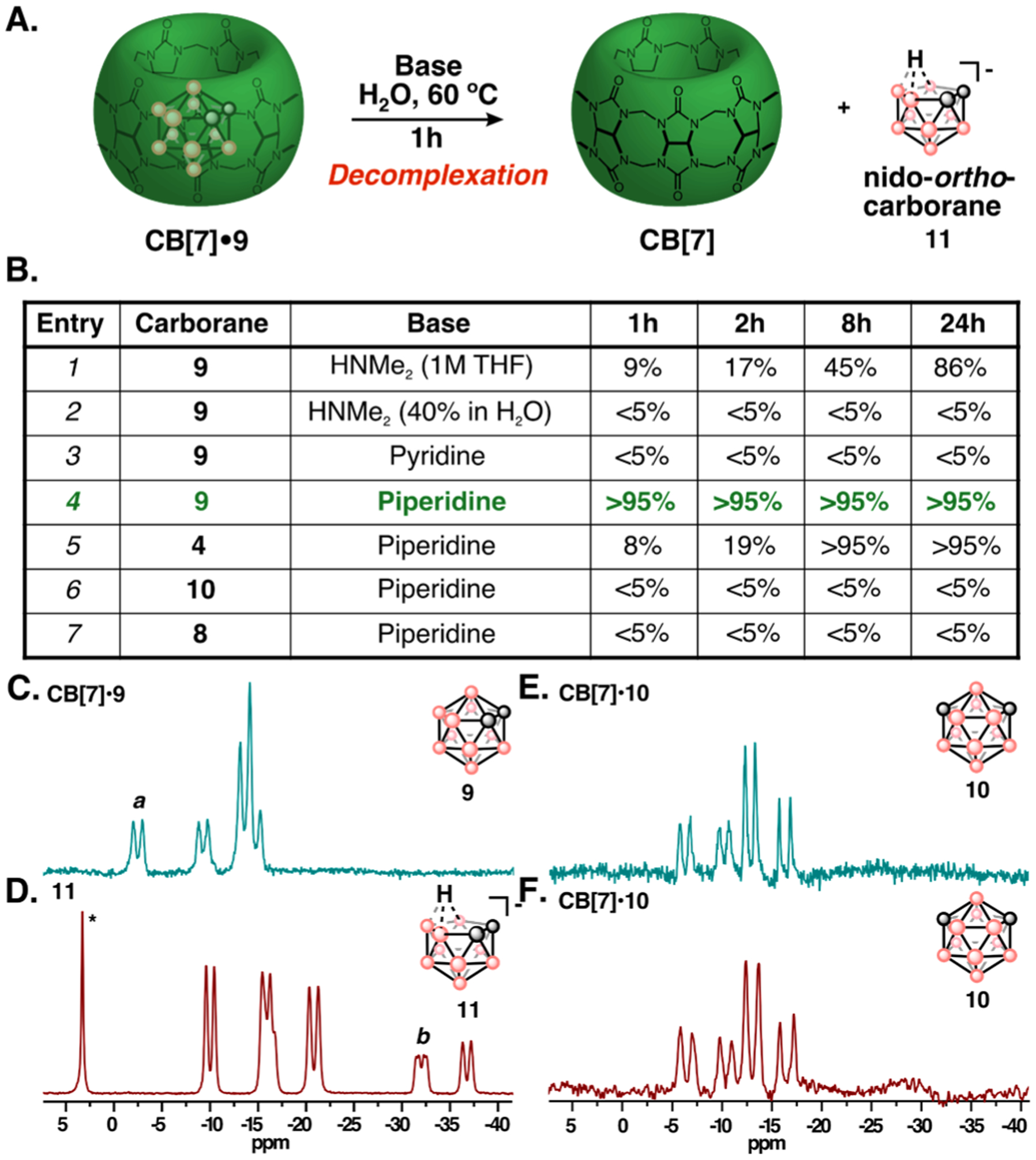
(A) Decomplexation of the CB[7]•9 complex through deboronation of ortho-carborane (9) with base to yield nido-ortho-carborane (11) and free CB[7]. (B) Conditions screened to evaluate the decomplexation of CB[7]•carborane complexes. CB[7]•carborane and base (5 equiv.) were combined in H2O, stirred at 60 °C, and monitored by 11B-NMR spectroscopy. Generation of 11 was calculated by relative integration of baseline corrected 11B-NMR spectra (Figure S9–S13, Table S1) (C-F) 11B-NMR spectra taken before (C) and after (D) 1h of subjecting CB[7]•9 complex (C,D) or CB[7]•10 complex (E,F) to 20% piperidine/H2O (v/v) at 60 °C. a, b denote the peaks used for relative integration measurements. *denotes borate side-product known to form during deboronation of 9.
