Scheme 2.
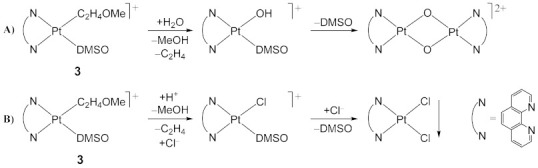
(A) Hydrolysis in water of the [Pt(η1-C2H4OMe)(DMSO)(phen)]+ (3) complex, involving the σ-C2H4OMe moiety, providing the reactive [Pt(DMSO)(OH)(phen)]+ intermediate, followed by slow DMSO loss and formation of the [{Pt(μ-OH)(phen)}2]2+ hydroxo-bridged dimer. The last step is disfavored by a high DMSO concentration. (B) Parallel mechanism of water hydrolysis for complex 3, favored by high Cl− concentration, providing the reactive [PtCl(DMSO)(phen)]+ intermediate, producing by DMSO loss, the low soluble [PtCl2(phen)] species.
