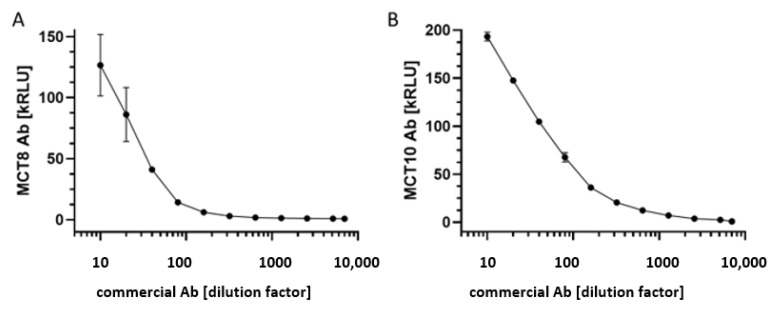
Figure 1.
Characterization of the novel assays by a commercial anti-MCT8 or anti-MCT10 antiserum. (A) Dilution of the commercial MCT8-Ab with human serum yielded dose-dependent signals, indicating a suitable assay design and acceptable measuring range for clinical samples. (B) The commercial antiserum to human MCT10 was similarly suitable for testing the newly generated assay for detecting and quantifying MCT10-aAb in clinical samples. Measurements were conducted in duplicates, kRLU; 1000 relative light units.