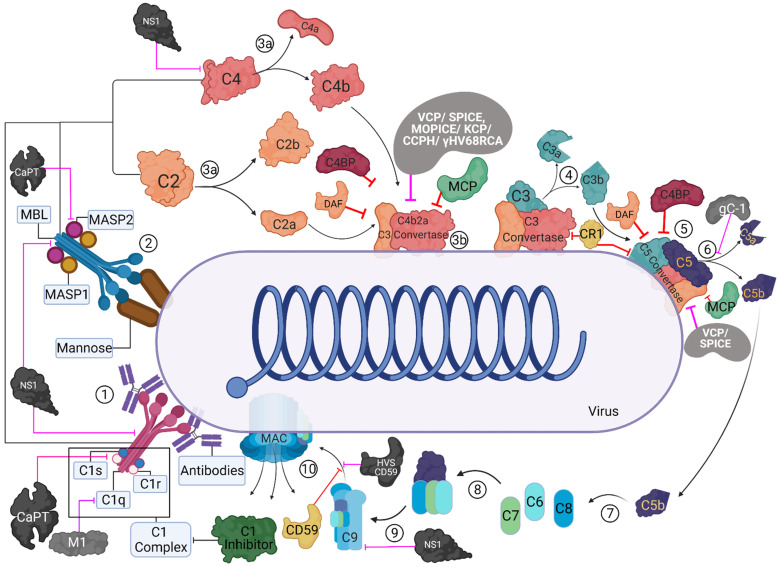
Figure 1.
Activation and regulation of the classical and lectin pathways and their targeting by virally encoded molecules. In the classical pathway (CP), C1 complex recognizes the antigen-antibody complexes present on the viral surface (1). In the lectin pathway (LP), MBL/ficolin-MASP complexes can recognise other carbohydrate patterns on the surfaces of viruses (2). Upon activation, these complexes can cleave C4 and C2 (3a) that can lead to the formation of C4bC2a (CP/LP C3 convertase) (3b). The C3 convertase further cleaves C3 into C3b and C3a; C3b is known to opsonise the viral surfaces, whereas C3a can lead to an enhanced acquired immune responses (4). C3b-C3 convertase interaction can generate C5 convertase (5), which cleaves C5 into C5b and C5a (6). C5b further interacts with C6 and C7 (C5b-7) (7) that can bind to the viral surface, while C5a induces further infiltration. C5b-7 then binds to C8, which can generate C5b-8 that penetrates the membrane (8). Finally, the C9 binds to the C5b-8 and results in MAC formation leading to the virolysis (10). These activation pathways are regulated at different steps by host complement regulators such as C1 inhibitor, C4b-binding protein (C4BP), complement receptor 1 (CR1; CD35), membrane cofactor protein (MCP; CD46), decay-accelerating factor (DAF; CD55), and CD59. Viral proteins that target these pathways are: Vaccinia virus complement control protein (VCP), Smallpox inhibitor of complement enzymes (SPICE), Monkeypox inhibitor of complement enzymes (MOPICE), Kaposi’s sarcoma-associated herpesvirus inhibitor of complement activation (KCP), Murine gamma-herpesvirus 68 regulator of complement activation (γ-HV68 RCA), Herpesvirus saimiri complement control protein homologue (CCPH), Herpesvirus saimiri CD59 homologue (HVS CD59), Flavivirus non-structural protein 1 (NS1), HSV-1 glycoprotein C (gC-1), human astrovirus coat protein (CoPt), and Influenza virus matrix protein 1 (M1). These are identified as black/grey protein with white text, and pink inhibitory arrows mark the regulator they inhibit.