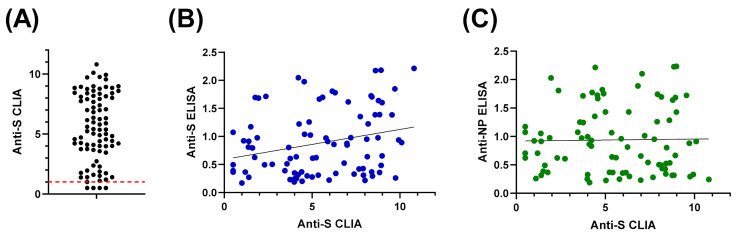
Figure 5.
Compairson and correaltion of COVID-19 NP and S IgG ELISAs with FDA-approved CLIA. (A) The result obtained from CLIA for all samples that were tested positive by MN assay, S- and NP-based ELISAs. (B) Signficant correlation between CLIA and S-based ELISA; r2 = 0.2595 and p value = 0.015. (C) Lack of correlation between CLIA and NP-based ELISA; r2 = 0.0167 and p value = 0.879.