Optimization of reaction conditionsa.
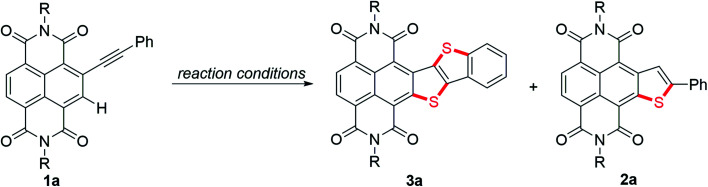
| |||
|---|---|---|---|
| Entry | Reaction conditions (reagent (equiv)) | % Yield of 3ab | % Yield of 2ab |
| 1 | K2S (6), 120 °C | 51 | 19 |
| 2 | K2S (6), 140 °C | 66 | 16 |
| 3 | K2S (4), 140 °C | 42 | 44 |
| 4 | K2S (2), 140 °C | 10 | 64 |
| 5c | K2S (6), H2O (30), 140 °C | 60 | 19 |
| 6 | Na2S·9H2O (6), 140 °C | 20 | 15 |
| 7 | S8 (6), 140 °C | 10 | 71 |
| 8 | S8 (6), Et3N (10), 140 °C | Trace | 98 |
| 9d | K2S (6), 140 °C | 83 | 16 |
Reactions were run in 0.03 mmol scale for 24 h in Ar (R = hexylheptyl).
Isolated yields of 2a and 3a.
40 h.
Before heating at 140 °C, the reaction mixture was stirred at 25 °C for 10 min.
