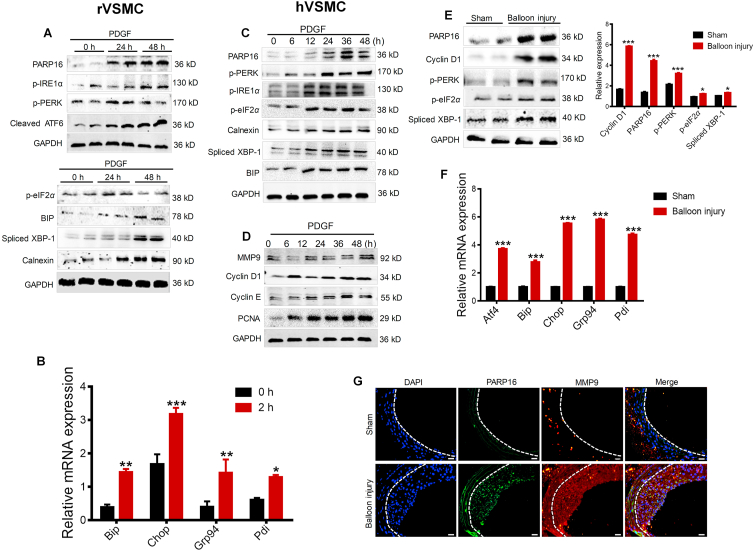
Figure 1.
PARP16 and ER stress are involved in SMC proliferation and migration as well as neointima formation. (A) rVSMCs were treated with PDGF-BB (20 ng/mL) for 24 and 48 h, cell extracts were collected for determining the protein levels of PARP16 and activation of three signaling branches of UPR: PERK, IRE1α/XBP-1 and ATF6 by Western blot. (B) mRNA levels of UPR target genes in rVSMCs after PDGF-BB treatment for 2 h. All data are represented as mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. 0 h, each acquired from three individual experiments. (C) The protein levels of PARP16 and activation of three signaling UPR branches PERK, IRE1α/XBP-1 and ATF6 were detected in hVSMCs after 20 ng/mL PDGF-BB stimulation for indicated time using Western blot; (D) Western blot analysis of MMP9, cyclin D1, cyclin E, PCNA protein levels in hVSMCs after 20 ng/mL PDGF-BB treatment. (E)–(G) PARP16 and ER stress were involved in intimal hyperplasia after rat carotid artery balloon injury. Western blot analysis of the PARP16, cyclin D1, p-PERK, p-IRE1α, spliced-XBP-1 protein levels in arteries from rat with sham operation or balloon injury for 14 days (E); mRNA levels of UPR target genes in arteries from rat with sham operation or balloon injury for 14 days (F); all data are represented as means ± SEM; ∗P < 0.05, ∗∗∗P < 0.001 vs. Sham group, n = 7 in each group. (G) Immunofluorescent staining with PARP16 (green) and MMP9 (red) in the rat carotid artery from the sham operation or 14-day post-balloon injury. All sections were counter-stained by DAPI to visualize nuclei (blue), scale bars: 100 μm.