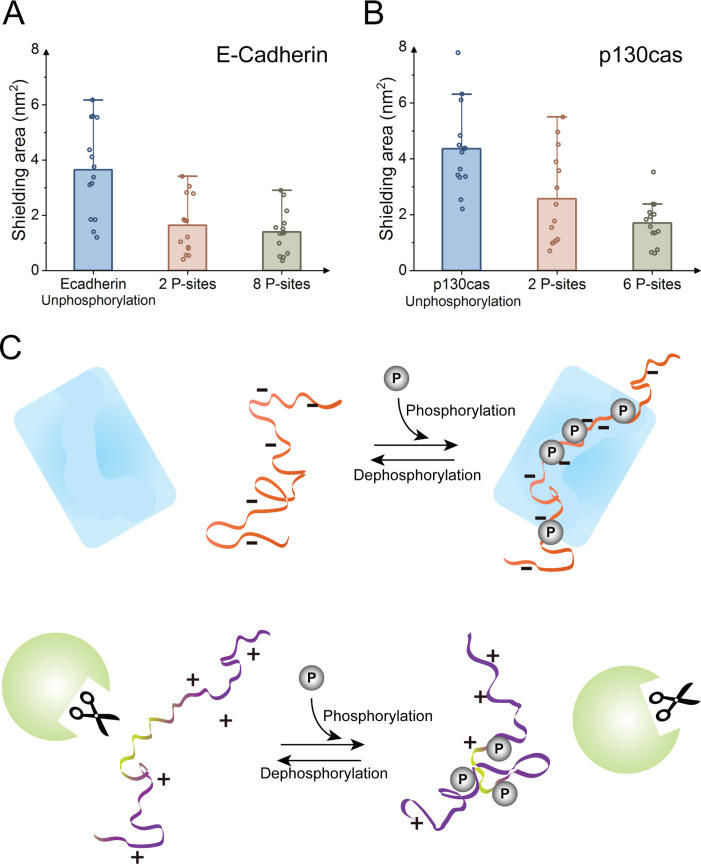
Fig 7. Interaction site exposure of E-Cadherin and p130Cas changes upon phosphorylation and implications for biological functions of IDPs.
Shielded area of interaction protein residues by intramolecular interactions for E-Cadherin (A) and p130Cas (B) for unphosphorylated states, biologically relevant two phosphorylation sites (2 P-sites) and multiple phosphorylation sites (8 P-sites for E-Cadherin and 6 P-sites for p130Cas). Data points: average along single 400 ns trajectories; bars: average over 14 trajectories, errors bars: standard errors of the mean of the 14 averages. (C) Schematic view of a defense-attack perspective: phosphorylation can cause functional changes of IDPs beyond direct recognition. Phosphorylation around negatively charged active site can expose binding sites, resulting in an extended and favorable binding conformation that more easily interacts with binding partners, while phosphorylation of positively (or dephosphorylation of negatively) charged active sites can reduce exposure to the environment and protect IDPs e.g. from proteolysis.