Abstract
BACKGROUND AND PURPOSE: Persistently hypointense lesions on T1-weighted MR images have been shown to correlate with the amount of axonal damage and clinical disability in multiple sclerosis (MS) patients. The purpose of this study was to investigate whether diffusion coefficient Dav and fractional anisotropy (FA) are able to detect quantifiable differences among three groups of focal nonactive multiple sclerosis (MS) lesions that appear qualitatively different on T1-weighted images.
METHODS: Conventional and diffusion tensor MR images of the brain were obtained in 18 patients with relapsing remitting (n = 10) or secondary progressive (n=8) MS and in 18 healthy volunteers. Focal nonactive MS lesions were classified as T1 isointense, T1 mildly hypointense, and T1 severely hypointense on unenhanced T1-weighted images. Differences among groups were assessed with one-way analysis of variance using the T1 lesion appearance as the grouping factor and either FA or coefficient Dav as the dependent measure.
RESULTS: FA was lowest and coefficient Dav was highest in T1 severely hypointense lesions, and then, in ascending and descending order, respectively, T1 mildly hypointense lesions and T1 isointense lesions. A significant difference in the values of FA was detected only between T1 isointense lesions and T1 severely hypointense lesions (P < .01), whereas no difference was found between T1 mildly hypointense lesions and either one of these two groups. A significant difference was found in the values of coefficient Dav among all investigated lesion groups. Coefficient Dav was found to correlate inversely with the T1-weighted contrast ratio (r=−0.58, P < .0001), whereas FA and ΔFA (percentage of FA variation in the lesion, a relative FA measure that minimizes the effect of FA spatial dependence) were not.
CONCLUSION: Coefficient Dav is more sensitive than FA to variations in the degree of T1 hypointensity and, thus, in the amount of the permanent brain tissue damage in patients with MS.
MR imaging is routinely used to confirm the diagnosis of multiple sclerosis (MS) and to monitor the course of the disease. Conventional T2-weighted images are very sensitive for identifying focal MS lesions, and T2-based lesion volumes are used to measure clinical severity (1). However, the correlation between the T2 lesion load and clinical disability is suboptimal, mainly because of the lack of histopathologic specificity of the T2-weighted images (2–4). Considering the correlation between MR imaging measures of brain atrophy and the level of clinical disability or the course of the disease, measurements of the whole brain volume have also been proposed as a marker of MS severity (5–9), but brain atrophy is a late finding, thereby implying that MR imaging measures of brain atrophy might not be sensitive to the most early and subtle evidences of tissue loss and disorganization. On the other hand, MR spectroscopy specifically identifies damaged axons by showing reduced levels of N-acetyl-aspartate (10), an amino acid localized only within neurons in the mature brain (11), but has limited spatial resolution.
Evidence has emerged from pathologic biopsy (12) and autopsy (13) studies that the degree of T1 hypointensity correlates with the amount of axonal damage and clinical disability (14). Change in T1 hypointense lesion load has therefore recently been recommended as a secondary outcome measure in phase III MS clinical trials (15). However, the full disease burden may be underestimated on T1-weighted images, because brain MS abnormalities extending beyond the boundaries of plaques escape direct visualization.
Previous studies based on diffusion-weighted MR imaging, a technique that uses molecular water diffusion to probe tissue microstructure (16), have shown increased water diffusion in the lesions and normal appearing white matter (NAWM) (17, 18) and the potential for diffusion measures to distinguish among MS lesions of different severity (19, 20). A more complete description of the diffusion properties of a given tissue requires computation of the full diffusion tensor imaging (DTI) (21), from which rotationally invariant quantities (ie, independent of patient position and acquisition method) can be derived. In addition to the magnitude of water “diffusivity,” DTI provides a measure of water motion directionality (anisotropy). Normal white matter tracts with coherently oriented fibers show a high degree of diffusion anisotropy, whereas decreased anisotropy can be expected in white matter disease states in which myelination or axonal integrity is disrupted, as in MS. Previous investigations have reported lower fractional anisotropy (FA) values in lesions compared with NAWM (22–24), with a gradient of decreasing FA values from areas of NAWM remote from plaques, to areas of NAWM surrounding the plaques, to the plaques (25). To the best of our knowledge, only a few previously published studies have involved the specific assessment of anisotropy in focal nonactive MS lesions with different degrees of T1 hypointensity. These previous investigations have reported lower FA values in T1 hypointense than in T1 isointense lesions (24, 26, 27).
This study was designed to determine whether DTI-based measures are able to detect quantifiable differences among three groups of focal nonactive MS lesions that appear qualitatively different on T1-weighted images and among various white matter regions in patients with MS and normal volunteers and to determine whether DTI-based measures correlate with the degree of lesion T1 hypointensity.
Methods
Phantom Experiments
As a measure of quality, the DTI sequence was preliminarily tested on a phantom of water doped with NiSO4 to compare our diffusion and anisotropy measurements with reference values of the literature.
Participants
Twenty-six patients with clinically definite MS (28) were sequentially enrolled in the study between February and August 2000. Of these, 18 (10 with relapsing-remitting disease, eight with secondary progressive disease) fulfilled the inclusion criteria. To be included in the study, patients could not have received immunosuppressive, immunoregulatory, or steroid therapy for at least 6 months before study initiation. They also could not have had a relapse during the 3 months preceding the study. Patients with relapsing-remitting disease (four men and six women; mean age, 26 years; age range, 18–37 years) had had clinically definite MS for at least 2 years with a relapsing-remitting course. The mean age at onset of disease was 23 years (range, 16–32). Expanded Disability Status Scale score was 3 (range, 1.0–5). Patients with secondary progressive MS (four men and four women; mean age, 38 years; age range, 29–46 years) had experienced a slowly progressive increase in disability for at least 6 months, with or without superimposed relapses, after an initial relapsing-remitting course (29). The mean age at disease onset was 26 years (range, 18–37), and mean duration of the relapsing-remitting phase was 11 years (range, 3–25). Expanded Disability Status Scale score was 6.5 (range, 4.5–7.5). Eighteen sex- and age-matched healthy volunteers (eight men and 10 women; mean age, 29.3 years; range, 20–48 years) served as control participants. The study was approved by the local ethics committee, and all participants provided written informed consent before study initiation.
MR Data Acquisition
Both the patients and the volunteers underwent a combined conventional and diffusion tensor MR imaging protocol. A Siemens Vision 1.5-T whole body unit (Erlangen, Germany), equipped with echo-planar capability, and a circularly polarized 270-mm-diameter head coil were used for image acquisition. The MR imaging protocol included the following: 1) axial view T1-weighted spin-echo (650/14 [TR/TE]; flip angle, 70 degrees); 2) axial view dual-echo T2-weighted turbo spin-echo (3800/22, 90[TR/first TE, second TE]; echo train length, 5); 3) axial view DTI single shot echo-planar pulse sequence (5000/98; six b values ranging from 0 to 530 s/mm−2, with the gradients used for b ≠ 0 applied in six noncollinear directions); and 4) contrast-enhanced (IV administered 0.1 mmol/kg gadopentetate dimeglumine) axial view T1-weighted spin-echo (650/14; flip angle, 70 degrees) imaging. For the sequences described in imaging protocols 1, 2, and 4, 21 contiguous sections were acquired with 5-mm thickness, 256 × 256 matrix, and 240 × 240 field of view (resolution, 0.94 × 0.94 × 5 mm). The sections were positioned to run parallel to the anterior commissure–posterior commissure line and to cover the whole brain. For the DTI sequence, 15 axial view sections with 5-mm thickness, 128 × 128 matrix, and 240 × 240 field of view (resolution, 1.88 × 1.88 × 5 mm) were acquired by positioning the sections to match exactly the 15 central sections of the conventional images. The DTI sequence is implemented with self-correction for eddy current effects by using both compensation gradients and phase correction (30). The total examination time was approximately 20 minutes.
MR Imaging Data and Statistical Analysis
Collected MR imaging studies were transferred via Ethernet to an independent workstation (Silicon Graphics SGI) running a homemade MR image processing software implemented under MATLAB 5.3.1 (Mathworks). After pixel-by-pixel diagonalization of the diffusion tensor matrix, maps of the mean diffusivity coefficient Dav (equal to one-third of the trace) and maps of FA were derived. FA is a robust estimator of diffusion anisotropy that is able to map diffusion anisotropy with the greatest detail and signal-to-noise ratio compared with other anisotropy indices, such as relative anisotropy and volume ratio (31). Values for FA, a unitless ratio of diffusion coefficients, range from 0 to 1, where 0 represents isotropic diffusion and 1 represents diffusion in a single direction. T2-weighted and proton density–weighted images were analyzed for lesion identification by one experienced reader (observer 1). Based on their appearance on contrast-enhanced T1-weighted images, 12 enhancing lesions were identified and excluded from further analysis. With guidance from T2- and proton attenuation-weighted images, regions of interest of uniform shape and size (approximately eight pixels corresponding to 28 mm2, given a pixel size of 1.88 × 1.88 mm) were outlined in the plaques on the non-diffusion-weighted b0 (b value=0) images of the DTI dataset and subsequently projected onto the coefficient Dav and FA maps. Plaques <15 mm2 (approximately four pixels) were excluded. Likewise, equivocal areas adjacent to ventricular surfaces were not considered. A total of 83 plaques were initially assessed in 18 patients.
Regions of interest recorded on the images with no diffusion weighting (b=0) were transferred to the T2-weighted images and were manually adjusted, considering the lack of spatial correspondence between diffusion- and T2-weighted images, to fit into corresponding lesions. The same regions of interest were also used to generate matching regions of interest on T1-weighted images. Two other experienced readers (A.C.-S., F.F.), blinded to the diffusion and anisotropy data, patient’s identity, and clinical status, assessed by consensus the degree of hypointensity of the plaques selected on unenhanced T1-weighted images in comparison with NAWM and gray matter, as previously described (13). T1 isointense lesions had the same signal intensity (SI) as that of NAWM, T1 mildly hypointense lesions were of SI equal to or higher than that of gray matter, and T1 severely hypointense lesions were of SI lower than that of gray matter (Fig 1). To correct for random variation in overall SI among examinations, analysis of the selected lesions also included calculation of the contrast ratio on T1-weighted images. The contrast ratio (SIlesion/SICSF) was defined as the SI of a lesion divided by the SI of CSF, analyzed in the same section as the investigated lesion.
Fig 1.
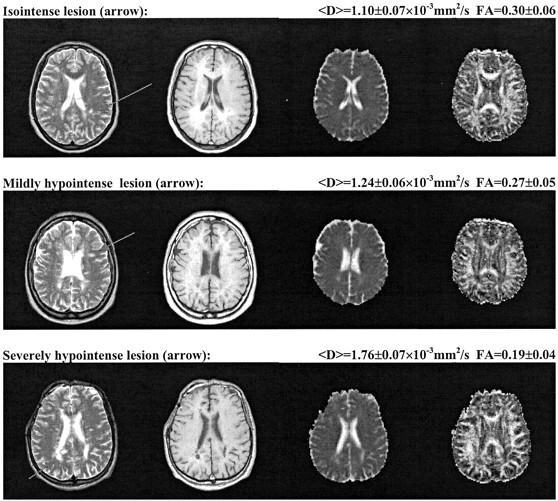
Three examples of MS lesions (arrows) with different T1 appearance. Top, isointense lesion (FA=0.30 ± 0.06, coefficient Dav=1.10 ± 0.07 × 10−3 mm2/s); middle, mildly hypointense lesion (FA=0.27 ± 0.05, coefficient Dav=1.24 ± 0.06 × 10−3 mm2/s); bottom, severely hypointense lesion (FA=0.19 ± 0.04, coefficient Dav = 1.76 ± 0.07 × 10−3 mm2/s). For each row, from left to right: axial view fast spin-echo T2-weighted image, spin-echo T1-weighted image, coefficient Dav map, and FA map.
For each region of interest in the lesions, a region of interest of identical size was drawn in a symmetric area of NAWM in the contralateral hemisphere. The NAWM region paired with each plaque was at the closest feasible location as the plaque. If the matching NAWM region in the contralateral hemisphere showed abnormal SI, it was discarded together with the corresponding plaque. After removal of seven plaques, a total number of 76 lesions and 76 NAWM regions in the contralateral hemisphere were assessed. To minimize the effect of FA variations due to normal regional differences in the brain architecture, relative FA variation was computed for each plaque by using as reference the contralateral NAWM region. Specifically,
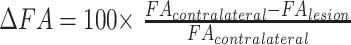 |
where FAlesion is the FA of the lesion, and FAcontralateral is the FA of the NAWM region symmetric to the lesion in the contralateral hemisphere. Finally, for each lesion, a corresponding area of normal white matter was selected in the sex- and age-matched volunteers by choosing the section that most closely matched with the section in the patient, thereby obtaining 76 regions of interest. The mean values ± SD for FA and coefficient Dav were recorded for each region of interest placed in the plaques, NAWM, and normal white matter.
A paired Student’s t test was used to assess differences on the values of absolute FA and coefficient Dav between the following pairs: plaques and NAWM regions, plaques and normal white matter regions, and NAWM regions and normal white matter regions. The paired Student’s t test was used because of the intrinsic region-related FA variation, which required pairing of each value in the plaque with its analogous area in the matched NAWM or normal white matter region. To account for multiple comparisons, data were analyzed by conducting one-way analysis of variance using a model with the T1-based lesion appearance (T1 isointense, T1 mildly hypointense, and T1 severely hypointense) as the grouping factor and either absolute FA, coefficient Dav, or ΔFA (a relative FA measure that minimizes the effect of FA spatial dependence) as the dependent measure. Analysis of variance was applied after verifying that the variables adhered to a normal distribution by using Kolmogorov-Smirnov tests and was followed by Bonferroni post hoc comparisons.
The values of FA, coefficient Dav, and ΔFA were then correlated with the SIlesion/SICSF ratio by calculating the Pearson correlation coefficient. The level for statistical significance was set at P < .05.
Results
Phantom Experiments
In quantitative diffusion tensor measurements of phantom of doped water, coefficient Dav and FA, computed on regions of interest of size comparable with those used for patients, were found to be 2.28 ± 0.03 (× 10−3 mm2/s) and 0.05 ± 0.02, respectively. These measured quantities are in close agreement with previously published data (27).
Participants
DTI measurements yielded high quality data for both patients with MS and healthy volunteers. Of the 76 nonenhancing lesions, 25 were isointense (80% on images of patients with relapsing-remitting disease and 20% on images of patients with secondary progressive disease), 26 were mildly hypointense (40% on images of patients with relapsing-remitting disease and 60% on images of patients with secondary progressive disease), and 25 were severely hypointense (30% on images of patients with relapsing-remitting disease and 70% on images of patients with secondary progressive disease) on unenhanced T1-weighted images.
DTI Measures in Various White Matter Regions
Table 1 summarizes the mean value (± SD) for FA and coefficient Dav as measured in MS plaques, NAWM regions, and normal white matter regions. The lowest FA values were measured in plaques, and then, in ascending order, in NAWM and normal white matter. By contrast, coefficient Dav values were highest in plaques, intermediate in NAWM, and lowest in normal white matter. On average, a 13% decrease in FA and an 11% increase in coefficient Dav occurred in NAWM compared with normal white matter, and a 31% decrease in FA and a 35% increase in coefficient Dav occurred in plaques compared with NAWM. The Student’s t tests revealed significant differences in the values of FA and coefficient Dav for all paired comparisons [plaques and NAWM regions (FA, P < .0001; coefficient Dav, P < .0001); plaques and normal white matter regions (FA, P < .0001; coefficient Dav, P < .0001); NAWM and normal white matter regions (FA, P < .001; coefficient Dav, P < .0001)].
TABLE 1:
Mean values (± SD) for fractional anisotropy and coefficient Dav in the various investigated white matter brain regions
| FA | Dav (×10−3 mm2/s) | |
|---|---|---|
| All plaques (n = 76) | 0.22 ± 0.07 | 1.54 ± 0.33 |
| NAWM regions (n=76) | 0.32 ± 0.09 | 1.00 ± 0.14 |
| NWM regions (n=76) | 0.37 ± 0.09 | 0.89 ± 0.08 |
Note.—FA indicates fractional anisotropy; Dav, average diffusivity; NAWM, normal appearing white matter; NWM, normal white matter. Note that FA is expressed as a unitless number that may range from 0 to 1 and that Dav is equal to one-third of the trace of the diffusion tensor.
The P value was <.001 for all paired t tests between groups (plaques and NAWM regions, plaques and NWM regions, NAWM and NWM regions).
Absolute FA, Coefficient Dav, and ΔFA Values in the T1-Based Lesion Groups
Table 2 summarizes the mean value (± SD) for FA, coefficient Dav, and ΔFA as measured in the group of T1 isointense, T1 mildly hypointense, and T1 severely hypointense lesions. The lowest FA values were measured in the T1 severely hypointense lesion group, and then, in ascending order, in the T1 mildly hypointense group and T1 isointense group. By contrast, coefficient Dav values were highest in the T1 severely hypointense lesion group, intermediate in the T1 mildly hypointense group, and lowest in the T1 isointense group. On average, a 16% reduction in FA and a 16% increase in coefficient Dav occurred when moving from the T1 isointense lesion group to the T1 mildly hypointense lesion group and a 9% reduction in FA and a 14% increase in coefficient Dav occurred when moving from the T1 mildly hypointense lesion group to the T1 severely hypointense lesion group. One-way analysis of variance, with appearance of MS lesions on T1-weighted images as the grouping factor, revealed an overall effect of the T1-based lesion type, both for FA (P < .01) and coefficient Dav (P < .0001). However, post hoc contrast analysis disclosed a significant difference in the values of FA only between the T1 isointense group and the T1 severely hypointense lesion group (P < .01) but not between T1 mildly hypointense lesions and either one of these two groups. A significant difference was found in the values of coefficient Dav among all investigated lesion groups (T1 severely hypointense > T1 mildly hypointense > T1 isointense). No significant effects of the T1-based lesion type were found in the values of ΔFA.
TABLE 2:
Mean values (± SD) for absolute and relative fractional anisotropy and coefficient Dav in the three groups of lesions of increasing hypointensity on the T1-weighted images
| FA | Dav (×10−3 mm2/s) | ΔFA | |
|---|---|---|---|
| T1 isointense (n = 25) | 0.26 ± 0.07 | 1.29 ± 0.17 | 19 ± 4 |
| T1 mildly hypointense (n=26) | 0.21 ± 0.06 | 1.54 ± 0.31 | 23 ± 6 |
| T1 severely hypointense (n=25) | 0.19 ± 0.06 | 1.80 ± 0.32 | 36 ± 4 |
Note.—FA indicates fractional anisotropy; Dav, averaged diffusivity; ΔFA, percentage FA variation in the lesion, obtained in relation to a symmetric normal appearing white matter area in the contralateral hemisphere.
Correlations between SIlesion/SICSF Ratio and Either FA, Coefficient Dav, or ΔFA Values
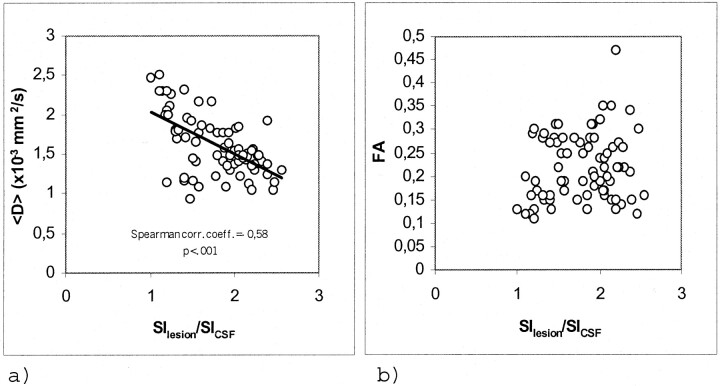
Considering all MS plaques, an inverse correlation was found between coefficient Dav and the T1-weighted SIlesion/SICSF ratio (r=−0.58; P < .001) whereas no correlation was found between either FA or ΔFA and the SIlesion/SICSF ratio (Fig 2).
Fig 2.
Scatter plots for the 76 lesions studied.
A, Coefficient Dav versus SIlesion/SICSF ratio. Significant inverse correlation was shown.
B, FA versus SIlesion/SICSF ratio. No correlation was shown.
Discussion
Although MS is primarily a demyelinating disease, axonal damage and loss have been documented from the beginning of MS research (32) and have been confirmed recently by several histopathologic studies (33, 34). Axonal loss may be an early event in MS lesion development (33, 34), independent, to some extent, of demyelinating activity (35). It is the prevailing process in chronic lesions, accounting for most of the patient’s permanent disability. In a previous study that investigated the histopathologic correlate of MS lesions on postmortem MR images of fresh brain material, axonal depletion was approximately 25% in T1 isointense lesions, 50% in T1 mildly hypointense lesions, and 70% in T1 severely hypointense lesions, with a highly significant correlation between degree of hypointensity on T1-weighted images and percentage of residual axons (13). In a more recent study that used neurofilament immunohistochemistry, it has been shown that at least two-thirds of the axons are lost in inactive, chronic MS lesions in the cervical spinal cord of patients with the secondary progressive form of MS (36). Several other pieces of evidence support the hypothesis that persistently hypointense lesions on T1-weighted images are the MR equivalent of axonal loss, including the decreased levels of N-acetyl-aspartate in T1 hypointense lesions shown by spectroscopic studies (11) and the correlation of T1 hypointense lesion load with clinical disability and disease progression (14, 37).
DTI Measures of Focal Nonactive MS Lesions
In this study, we used the degree of hypointensity on T1-weighted images as a marker of matrix destruction and axonal loss in patients with MS and analyzed the ability of DTI-based parameters to detect differences among three groups of nonactive MS lesions with increasing T1 hypointensity. We found that coefficient Dav values increased and FA values decreased with increasing T1 hypointensity. The mean values of coefficient Dav were significantly different among the three T1-based groups of lesions, thus allowing differentiation among them. More importantly, a correlation was found between coefficient Dav and the SIlesion/SICSF contrast ratio, a semi-quantitative measure of the degree of T1 hypointensity in lesions. This finding suggests that both MR parameters reflect the extent of axonal damage in patients with MS. In contrast to coefficient Dav, the mean values of FA were not significantly different among the three T1-based lesion groups, and a significant difference emerged only when the two extreme lesion categories (T1 isointense and T1 severely hypointense) were considered. Moreover, no correlation was found between FA values and the SIlesion/SICSF contrast ratio in lesions, a finding bringing further evidence to the concept that FA and the degree of T1 hypointensity provide at least partially independent information. One possible explanation is that measured FA values in the brain of patients with MS not only reflect the structural changes determined by the disease process but also the point-by-point variation of diffusion anisotropy within the normal brain (38). Brain white matter areas in which the fibers are incoherently oriented, such as the centrum semiovale or the U fibers, have normally relatively low FA values even though they are highly myelinated (21). Thus, FA may not be significantly reduced by MS lesions involving these regions.
To go some way toward the limitation of the regional dependence of diffusion anisotropy, we also conducted a statistical analysis on the values of ΔFA, a relative FA measure that minimizes the effect of FA spatial dependence. Just like absolute FA, relative FA values did not show correlation with the SIlesion/SICSF contrast ratio nor did they enable discrimination among the three T1-based lesion categories. We therefore conclude that coefficient Dav may be more sensitive than FA in determining the degree of axonal damage in patients with MS.
Comparisons with Previous DTI Studies
Previous studies (17–20, 22, 25, 39, 40) have reported increased coefficient Dav and decreased FA in MS plaques compared with NAWM, with observed values in the ranges of 0.90 to 1.59 (× 10−3 mm2/s) and 0.23 to 0.50, respectively. The mean coefficient Dav value of 1.54 (× 10−3 mm2/s) and the mean FA value of 0.22 measured in plaques in the current investigation are, with few exceptions (26), similar to those reported in most the above mentioned DTI studies (22–25). Moreover, some of these previous studies (23, 24, 26) have reported lower FA values in T1 hypointense than in T1 isointense lesions, a finding in accordance with our results when considering the T1 isointense and the T1 severely hypointense lesions only. Our mean FA measurements of 0.26 in T1 isointense lesions are very similar to the value of 0.27 reported by Filippi et al (24) but lower than the values of 0.43 and 0.51 reported by Bammer et al (27) and Werring et al (26), respectively. In only one previous study (27) were areas of extensive tissue destruction studied by means of DTI indices, and the reported FA value of 0.23 is very close to the value of 0.19 we found in the group of T1 severely hypointense lesions.
DTI Measures in Widespread Subtle Pathologic Abnormality
In agreement with previous investigations (39, 41, 42), we found significantly increased coefficient Dav and decreased FA values in NAWM compared with normal white matter, a finding supporting the idea that MS is a widespread disease able to cause microscopic changes that extend well beyond the margins of the focal lesions (43). Histopathologic and biochemical studies have reported astrocytosis, demyelination, perivascular inflammation, and increased water content in the NAWM of patients with MS (44, 45). More recently, substantial loss of axons in the NAWM has been suggested by spectroscopic studies (46, 47), MR imaging measures such as T1 and T2 TRs (48), and magnetization transfer imaging (49) and has been confirmed by direct quantitation of axonal attenuation and volume depletion on postmortem MS brains (50). Several possible mechanisms could account for the extensive loss of axons in the NAWM, including denervation by transection in remote lesions (33, 34) and effects of diffusable neurotoxic factors from lesions (51). Because axonal damage in NAWM may have functional consequences for patients with mild disability early in the disease course, in vivo quantification of disease burden has several important implications, both in drug trials and patient treatment.
Limitations of the Study and Conclusions
The rationale for this study design is based on previous studies that have attempted to correlate appearance on T1-weighted images with pathologic findings (13). Based on these correlation studies, the assumption was made that the degree of T1 hypointensity and the extent of axonal loss are highly associated events in focal nonactive MS lesions. Under these circumstances, our data suggest that coefficient Dav may be more sensitive than FA in determining the degree of axonal damage in focal nonactive MS lesions. Thus, in contrast to coefficient Dav, the level of sensitivity of FA may not be sufficient for monitoring disease progression in patients with MS. Unfortunately, the lack of a direct correlation of DTI parameters with pathologic findings precludes firm conclusions regarding the pathologic specificity and sensitivity of DTI measures.
Considering the strong dependence of lesion FA changes on the preexisting normal local architecture of white matter (38), inferences regarding the underlying pathologic substrate should be made cautiously when absolute FA values are used. Although this spatial dependence can be diminished by calculating the relative FA variation, such calculation is not without limitations. Relating lesion FA to corresponding values in supposedly healthy regions of the contralateral hemisphere may lead to underestimation of FA changes because these regions may not be normal, as confirmed by the current study. In one previous investigation (27), lesion FA was related to areas of NAWM located in the vicinity of the lesion, and the resultant region-related FA measurements yielded a markedly reduced overlap between lesion types and normal white matter compared with absolute FA values. However, NAWM in the vicinity of MS lesions may be more profoundly altered than NAWM remote from lesions, as recently shown (25). Ideally, regional comparisons would benefit from spatial normalization and exact interpolation of patient’s anatomic structures onto an anisotropy-based atlas generated from volunteer studies (27).
Conclusion
In conclusion, this study shows abnormal coefficient Dav and FA values in both MS lesions and in NAWM. More importantly, this study suggests that the discriminatory power of coefficient Dav to distinguish focal nonactive MS lesions of different T1 hypointensity may be greater than that of FA. Should these results be confirmed by subsequent investigations, isotropic diffusion would probably qualify as a reliable, objective MR measure of axonal damage in patients with MS, thereby avoiding the more difficult, time-consuming, and technically demanding computation of FA.
Acknowledgments
We are grateful to Fernando Calamante, PhD, RCS Unit of Biophysics, Institute of Child Health, University of London, England, for having provided the diffusion sequence with self-correction for eddy currents. We also thank Caroline Castriota-Scanderbeg for help in reviewing the manuscript.
Footnotes
This work was supported by grant RF01.167 from the Ministero della Salute, Italy.
References
- 1.Simon JH, Jacobs LD, Campion M, et al. Magnetic resonance studies of intramuscular interferon beta-1a for relapsing multiple sclerosis: The Multiple Sclerosis Collaborative Research Group. Ann Neurol 1998;43:79–87 [DOI] [PubMed] [Google Scholar]
- 2.van Waesberghe JH, Castelijns JA, Scheltens P, et al. Comparison of four potential MR parameters for severe tissue destruction in multiple sclerosis lesions. Magn Reson Imaging 1997;15:155–162 [DOI] [PubMed] [Google Scholar]
- 3.Barnes D, Munro PM, Youl BD, Prineas JW, McDonald WI. The longstanding MS lesion: a quantitative MRI and electron microscopic study. Brain 1991;114:1271–1280 [DOI] [PubMed] [Google Scholar]
- 4.Newcombe J, Hawkins CP, Henderson CL, et al. Histopathology of multiple sclerosis lesions detected by magnetic resonance imaging in unfixed postmortem central nervous system tissue. Brain 1991;114:1013–1023 [DOI] [PubMed] [Google Scholar]
- 5.Losseff NA, Webb SL, O’Riordan JI, et al. Spinal cord atrophy and disability in multiple sclerosis: a new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996;119:701–708 [DOI] [PubMed] [Google Scholar]
- 6.Stevenson VL, Leary SM, Losseff NA, et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology 1998;51:234–238 [DOI] [PubMed] [Google Scholar]
- 7.Fox NC, Jenkins R, Leary SM, et al. Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI. Neurology 2000;54:807–812 [DOI] [PubMed] [Google Scholar]
- 8.Ge Y, Grossman RI, Udupa JK, et al. Brain atrophy in relapsing-remitting multiple sclerosis and secondary progressive multiple sclerosis: longitudinal quantitative analysis. Radiology 2000;214:665–670 [DOI] [PubMed] [Google Scholar]
- 9.Jagust WJ, Noseworthy JH. Brain atrophy as a surrogate marker in MS: faster, simpler, better? Neurology 2000;54:782–783 [DOI] [PubMed] [Google Scholar]
- 10.Brex PA, Parker GJ, Leary SM, et al. Lesion heterogeneity in multiple sclerosis: a study of the relations between appearances on T1 weighted images, T1 relaxation times, and metabolite concentrations. J Neurol Neurosurg Psychiatry 2000;68:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Walderveen MA, Barkhof F, Pouwels PJ, van Schijndel RA, Polman CH, Castelijns JA. Neuronal damage in T1-hypointense multiple sclerosis lesions demonstrated in vivo using proton magnetic resonance spectroscopy. Ann Neurol 1999;46:79–87 [DOI] [PubMed] [Google Scholar]
- 12.Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 1997;42:783–793 [DOI] [PubMed] [Google Scholar]
- 13.van Walderveen MA, Kamphorst W, Scheltens P, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 1998;50:1282–1288 [DOI] [PubMed] [Google Scholar]
- 14.van Walderveen MA, Barkhof F, Hommes OR, et al. Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology 1995;45:1684–1690 [DOI] [PubMed] [Google Scholar]
- 15.Simon JH, Lull J, Jacobs LD, et al. A longitudinal study of T1 hypointense lesions in relapsing MS: MSCRG trial of interferon beta-1a: Multiple Sclerosis Collaborative Research Group. Neurology 2000;55:185–192 [DOI] [PubMed] [Google Scholar]
- 16.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401–407 [DOI] [PubMed] [Google Scholar]
- 17.Christiansen P, Gideon P, Thomsen C, et al. Increased water self-diffusion in chronic plaques and in apparently normal white matter in patients with multiple sclerosis. Acta Neurol Scand 1993;87:195–199 [DOI] [PubMed] [Google Scholar]
- 18.Horsfield MA, Lai M, Webb SL, et al. Apparent diffusion coefficient in benign and secondary progressive multiple sclerosis by nuclear magnetic resonance. Magn Reson Med 1996;36:393–400 [DOI] [PubMed] [Google Scholar]
- 19.Nusbaum AO, Lu D, Tang CY, Atlas SW. Quantitative diffusion measurements in focal multiple sclerosis lesions: correlations with appearance on TI-weighted MR images. AJR Am J Roentgenol 2000;175:821–825 [DOI] [PubMed] [Google Scholar]
- 20.Castriota-Scanderbeg A, Tomaiuolo F, Sabatini U, Nocentini U, Grasso MG, Caltagirone C. Demyelinating plaques in relapsing-remitting and secondary-progressive multiple sclerosis: assessment with diffusion MR imaging. AJNR Am J Neuroradiol 2000;21:862–868 [PMC free article] [PubMed] [Google Scholar]
- 21.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–648 [DOI] [PubMed] [Google Scholar]
- 22.Griffin CM, Chard DT, Ciccarelli O, et al. Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler 2001;7:290–297 [DOI] [PubMed] [Google Scholar]
- 23.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol 1999;20:1491–1499 [PMC free article] [PubMed] [Google Scholar]
- 24.Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 2001;56:304–311 [DOI] [PubMed] [Google Scholar]
- 25.Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology 2002;222:729–736 [DOI] [PubMed] [Google Scholar]
- 26.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999;52:1626–1632 [DOI] [PubMed] [Google Scholar]
- 27.Bammer R, Augustin M, Strasser-Fuchs S, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med 2000;44:583–591 [DOI] [PubMed] [Google Scholar]
- 28.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231 [DOI] [PubMed] [Google Scholar]
- 29.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey: National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–911 [DOI] [PubMed] [Google Scholar]
- 30.Calamante F, Porter DA, Gadian DG, Connelly A. Correction for eddy current induced Bo shifts in diffusion-weighted echo-planar imaging. Magn Reson Med 1999;41:95–102 [DOI] [PubMed] [Google Scholar]
- 31.Papadakis NG, Xing D, Houston GC, et al. A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magn Reson Imaging 1999;17:881–892 [DOI] [PubMed] [Google Scholar]
- 32.Charcot J. Histologie de la sclerose en plaques. Gaz Hop (Paris) 1868;41:554–566 [Google Scholar]
- 33.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain 1997;120:393–399 [DOI] [PubMed] [Google Scholar]
- 34.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285 [DOI] [PubMed] [Google Scholar]
- 35.Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis: correlation with demyelination and inflammation. Brain 2000;123:1174–1183 [DOI] [PubMed] [Google Scholar]
- 36.Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain 2000;123:308–317 [DOI] [PubMed] [Google Scholar]
- 37.Truyen L, van Waesberghe JH, van Walderveen MA, et al. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 1996;47:1469–1476 [DOI] [PubMed] [Google Scholar]
- 38.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906 [DOI] [PubMed] [Google Scholar]
- 39.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 2001;56:926–933 [DOI] [PubMed] [Google Scholar]
- 40.Werring DJ, Clark CA, Droogan AG, Barker GJ, Miller DH, Thompson AJ. Water diffusion is elevated in widespread regions of normal-appearing white matter in multiple sclerosis and correlates with diffusion in focal lesions. Mult Scler 2001;7:83–89 [DOI] [PubMed] [Google Scholar]
- 41.Nusbaum AO, Tang CY, Wei T, Buchsbaum MS, Atlas SW. Whole-brain diffusion MR histograms differ between MS subtypes. Neurology 2000;54:1421–1427 [DOI] [PubMed] [Google Scholar]
- 42.Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol 2001;22:952–958 [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan S, Fu L, Pioro E, et al. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol 1997;41:385–391 [DOI] [PubMed] [Google Scholar]
- 44.Allen IV, McKeown SR. A histological, histo-chemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci 1979;41:81–91 [DOI] [PubMed] [Google Scholar]
- 45.Newcombe J, Cuzner ML, Roytta M, Frey H. White matter proteins in multiple sclerosis. J Neurochem 1980;34:700–708 [DOI] [PubMed] [Google Scholar]
- 46.Matthews PM, De Stefano N, Narayanan S, et al. Putting magnetic resonance spectroscopy studies in context: axonal damage and disability in multiple sclerosis. Semin Neurol 1998;18:327–336 [DOI] [PubMed] [Google Scholar]
- 47.De Stefano N, Narayanan S, Francis GS, et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol 2001;58:65–70 [DOI] [PubMed] [Google Scholar]
- 48.Miller DH, Johnson G, Tofts PS, MacManus D, McDonald WI. Precise relaxation time measurements of normal-appearing white matter in inflammatory central nervous system disease. Magn Reson Med 1989;11:331–336 [DOI] [PubMed] [Google Scholar]
- 49.Loevner LA, Grossman RI, Cohen JA, Lexa FJ, Kessler D, Kolson DL. Microscopic disease in normal-appearing white matter on conventional MR images in patients with multiple sclerosis: assessment with magnetization-transfer measurements. Radiology 1995;196:511–515 [DOI] [PubMed] [Google Scholar]
- 50.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 2000;123:1845–1849 [DOI] [PubMed] [Google Scholar]
- 51.Bo L, Dawson TM, Wesselingh S, et al. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol 1994;36:778–786 [DOI] [PubMed] [Google Scholar]