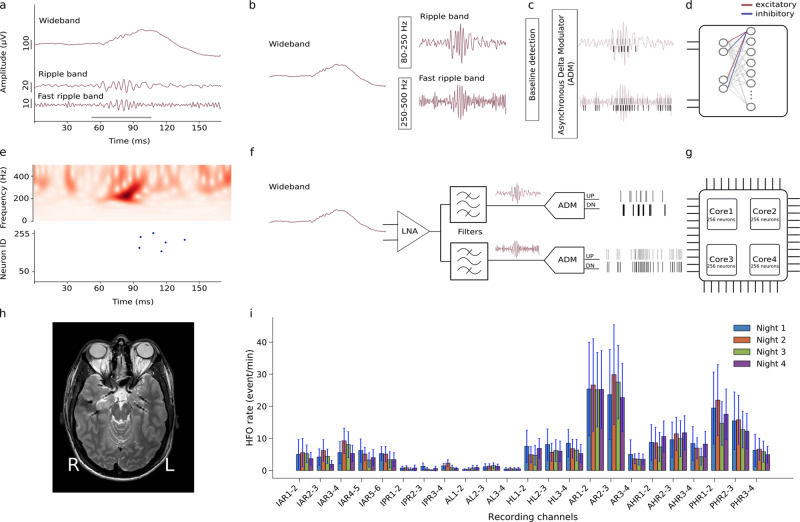
Fig. 1. Automatic HFO detection using a bio-inspired SNN.
a The pre-recorded iEEG signal in wideband, Ripple band (80–250 Hz) and Fast Ripple band (250–500 Hz). HFO stand out of the baseline in the signal. The period marked by the gray bar represents a clinically relevant HFO27,31. b–d Software simulated spiking neural network (SNN). For preprocessing, the wideband EEG is filtered in Ripple band and Fast Ripple band. A baseline detection stage finds the optimum threshold that is applied in an Asynchronous Delta Modulator (ADM) which converts the signal to spikes. Signal traces are encoded by UP spikes (gray bars) and DOWN spikes (black bars), which are then fed as input into the SNN. The SNN is implemented as a two-layer spiking network of integrate and fire neurons with dynamic synapses. Each neuron in the second layer receives four inputs: two excitatory spike trains from UP channels and two inhibitory ones from DOWN channels. The parameters of the network were chosen to exhibit the relevant temporal dynamics and tune the neurons to produce output spikes in response to input spike train patterns that encode clinically relevant HFO. e, top Time-frequency spectrum of the Fast Ripple iEEG of panel a. e, bottom Firing of SNN neurons indicates the occurrence of the HFO. f Block diagram of the neuromorphic system input headstage. The headstage comprises a low noise amplifier (LNA), two configurable bandpass filters, and two ADM circuits that convert the analog waveforms into spike trains. g The spikes produced by the ADMs are sent to a multi-core array of silicon neurons that are configured to implement the desired SNN. h MRI with 7 implanted depth electrodes that sample the mesial temporal structures of a patient with drug-resistant temporal lobe epilepsy (Patient 1). i Rates of HFO detected by the neuromorphic SNN for recordings made across four nights for Patient 1. The variability of the HFO rates across intervals within a night is indicated by standard error bars. Recording channels AR1-2 and AR2-3 in the right amygdala showed the highest HFO rates which were stable over nights. Thus, the neuromorphic system would predict that a therapeutic resection, which should include the right amygdala, would achieve seizure freedom. Indeed, a resection including the right amygdala achieved seizure freedom for >1 year.