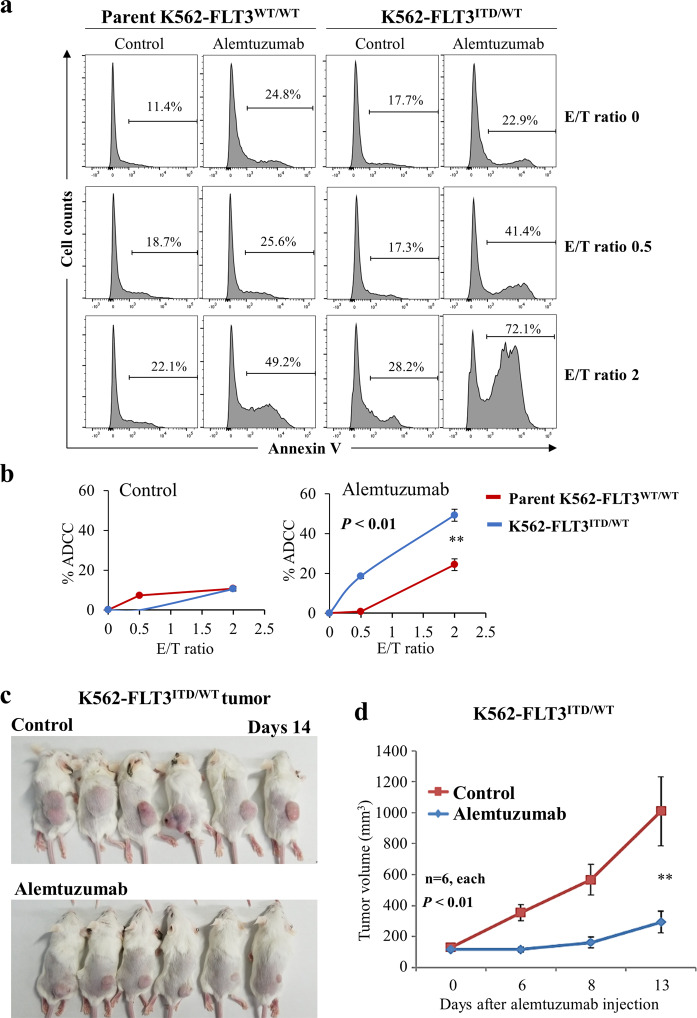
Fig. 6. Anti-tumor effects of alemtuzumab in K562–FLT3ITD/WT cells.
a Comparison of NK cell-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) with alemtuzumab in parent K562–FLT3WT/WT (left panel) and K562–FLT3ITD/WT (right panel) cells at the indicated E/T (effector cell/target cell) ratios. Phosphate-buffered saline (PBS) was used as control for alemtuzumab. b Percent ADCC by E/T ratio with control (left panel) and alemtuzumab (right panel). Red and blue lines indicate parent K562–FLT3WT/WT and K562–FLT3ITD/WT cells, respectively. Alemtuzumab showed higher percent ADCC in K562–FLT3ITD/WT cells than that in parent K562–FLT3WT/WT cells, whereas control did not. Data are expressed as mean ± SE (n = 3). c, d Effect of alemtuzumab on the tumor growth of xenografted K562–FLT3ITD/WT cells. c Mice implanted with tumors of xenografted K562–FLT3ITD/WT cells and treated with control (PBS) or alemtuzumab are pictured at the time of euthanasia on day 14. d Tumor volume of xenografted K562–FLT3ITD/WT cells treated with control or alemtuzumab on the indicated day. Data are expressed as mean ± SE (n = 6). Asterisks indicate statistically significant differences between control and alemtuzumab and K562–FLT3ITD/WT using two-tailed non-paired one-way analysis of variance (ANOVA), followed by post hoc Student’s t-test analysis. **p < 0.01.