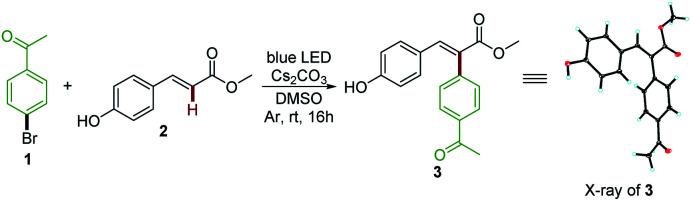
The model reaction and the reaction parameters evaluated.
| ||
|---|---|---|
| Entrya | Change from standard conditions | Yieldb (%) |
| 1 | None | 87 |
| 2 | No light | 0 |
| 3 | No light and heated to 80 °C | 0 |
| 4 | A 23 W CFL, instead of a blue LED | 56 |
| 5 | A 30 W white LED, instead of a blue LED | 50 |
| 6 | No base | 0 |
| 7 | KHCO3, instead of Cs2CO3 | 53 |
| 8 | Na2CO3, instead of Cs2CO3 | 40 |
| 9 | K2CO3, instead of Cs2CO3 | 73 |
| 10 | K3PO4, instead of Cs2CO3 | 43 |
| 11 | DBU, instead of Cs2CO3 | 51 |
| 12 | TMG, instead of Cs2CO3 | 54 |
| 13 | Et3N, instead of Cs2CO3 | 15 |
| 14 | DMF, instead of DMSO | 72 |
| 15 | CH3CN, instead of DMSO | 50 |
| 16 | Acetone, instead of DMSO | 66 |
| 17 | DCM, instead of DMSO | 18 |
Conditions: 1.0 equiv. of 4′-bromoacetophenone 1, 2.0 equiv. of methyl 4-hydroxycinnamate 2, 3.0 equiv. of base [0.10 M]; all solvents were rigorously degassed by freeze/pump/thaw cycles.
Isolated yields by chromatography.
