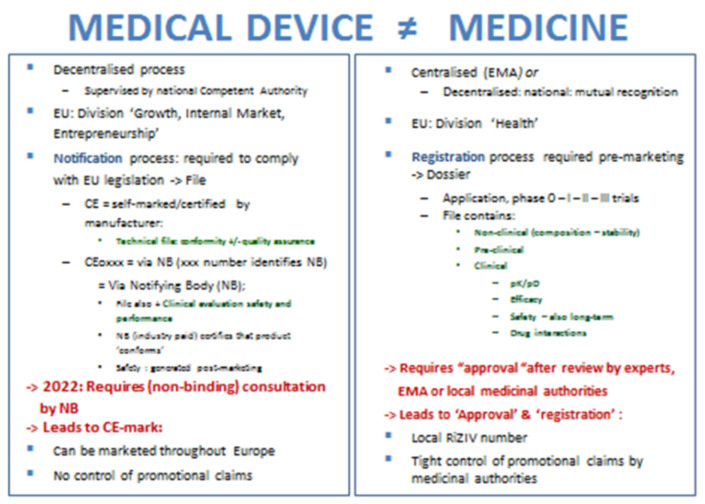
Figure 1.
Differences in regulations between medical devices and medicines in the European Union (definition of performance: “the ability of a device to achieve its intended purpose as stated by the manufacturer”; CE = Conformitè Europëeenne; NB: Notifying body; pK/pD = pharmacokinetics/pharmacodynamics); EEA = European Economic Area. EMA = European Medicines Agency; EU = European Union [3,4,5].