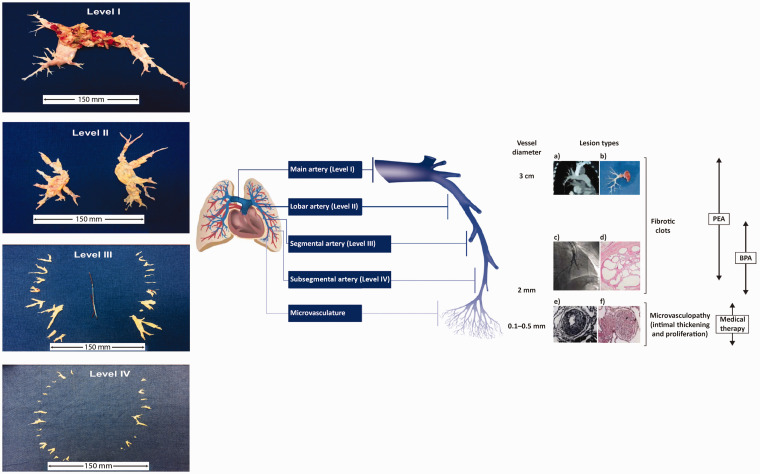
Fig. 2.
The management options for CTEPH target pathogenic manifestations in different parts of the pulmonary vascular bed. (a) Computed tomography scan of a pulmonary artery. (b) Organized fibrotic material removed during PEA. (c) Selective pulmonary angiogram of segmental and subsegmental pulmonary arteries, showing irregular vessel contour and occlusion, typical of CTEPH. (d) Microscopic examination showing a luminal filling defect with recanalized chronic thrombus (web lesion) and no evidence of vasculopathy in the subsegmental artery. (e) Intimal fibromuscular proliferation. (f) Plexiform lesion and vessel occlusion due to vasculopathy and proliferation. Adapted from Madani et al.5 [part (e) from Moser and Bloor33] and Madani.3 A schematic representation of a pulmonary artery is shown (note that vessel diameter is not to scale). PEA is used to remove thromboembolic lesions primarily in the proximal main artery (diameter of ∼1–3 cm), and lobar and segmental arteries;9,34 in expert surgical centers, lesions in distally located mid-segmental and subsegmental branches can be targeted by PEA,9 down to vessels of 2 mm in diameter. BPA mainly targets distal lesions in the segmental and subsegmental vasculature, down to small pulmonary arteries of 2–5 mm in diameter. Medical therapy targets microvasculopathy, including intimal thickening and fibromuscular proliferation, in vessels of 0.1–0.5 mm in diameter.35 Typical surgical specimens based on the most proximal level of obstruction are shown. The scale is in cm.
BPA: balloon pulmonary angioplasty; CTEPH: chronic thromboembolic pulmonary hypertension; PEA: pulmonary endarterectomy.