Figure 12.
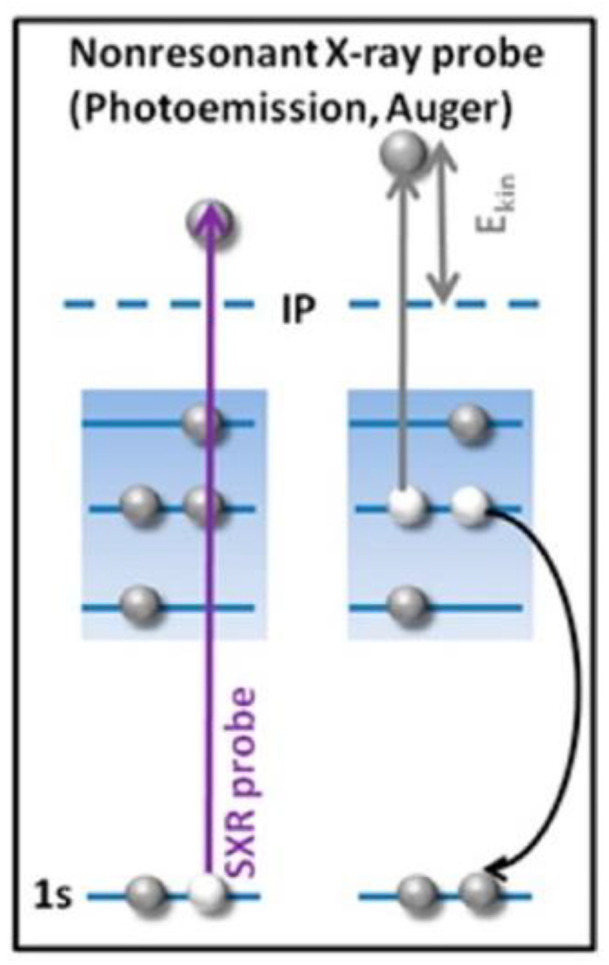
The Auger Effect, where we begin with a high-speed electron that knocks off an electron in the inner shell of an atom [157]. This leaves a vacant state (a 1s core hole), that is either filled by an upper electron that drops down to the inner shell, emitting a photon in the process (for heavy atoms, this energy is in the X-ray region, and thus results in X-ray fluorescence) or the excited ion relaxes by filling the core hole with an electron from a higher energy level, the resultant energy of this transition is taken up by an outer electron ejecting it from the atom, the Auger electron. The same is observed in the schematic where in non-resonant Auger spectroscopy, these vacancies are produced due to bombardment of a given sample with high energy electrons, in this case, a non-resonant X-ray pulse. Reprinted and adapted with permission from Ref [157] Copyright American Chemical Society (2016).
