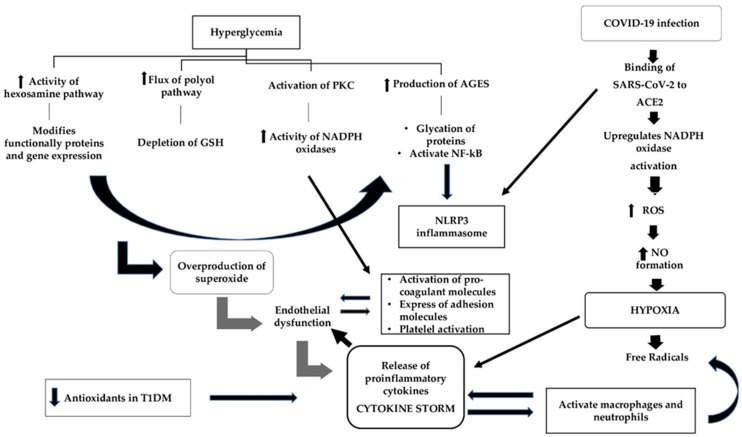
Figure 2.
The mechanisms of oxidative stress leading to adverse clinical outcomes during COVID-19 infection in patients with type 1 diabetes mellitus. In T1DM, hyperglycemia is a potent mediator of oxidative stress through four different pathways whose accumulative effect is the overproduction of superoxide, which leads to endothelial dysfunction and the subsequent release of pro-inflammatory cytokines. The destroyed endothelial cells release pro-coagulant molecules and express adhesion molecules. Simultaneously, hyperglycemia through the activation of the PKC pathway increases the expression of adhesion molecules, contributing additionally to endothelial dysfunction, and this leads to platelet activation and aggregation. During COVID-19 infection, the binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) results in the increased production of reactive oxygen species (ROS), which in turn increases NO formation and leads to cytopathic hypoxia. Hypoxia via the formation of free radicals upregulates the release of pro-inflammatory cytokines, which promotes endothelial derangement and contributes to pro-coagulability. Additionally, the upregulation of pro-inflammatory cytokines, in a vicious cycle, activate macrophages and neutrophils to produce more free radicals. Neutrophils have been recognized as pivotal mediators of severe COVID-19 disease by triggering the production of pro-inflammatory cytokines and maintaining the inflammatory state in the lungs. AGEs: advanced glycation end products; GSH: glutathione; NADPH: nicotinamide adenine dinucleotide phosphate; NF-kB: nuclear factor-kB; NLRP3: NOD-like receptor family pyrin domain-containing 3; NO: nitric oxide; PKC: protein kinase C; ROS: reactive oxygen species.