Figure 1.
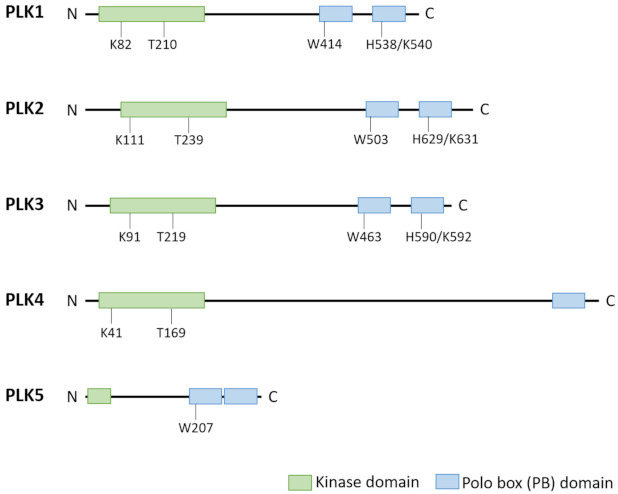
Structural differences between PLK family members PLK1–PLK5 [16]. PLK family members show a similar structure except PLK5. PLK1–PLK4 contain a highly conserved kinase domain at the N-terminal end and a non-catalytic polo box domain (PBD) at the C-terminus. The PBD is formed by three polo-boxes (PLK4) or two polo box motifs. These PBs are involved in substrate binding and regulation of kinase activity. Key residues of the kinase domain (acceptor lysine and T-loop threonine) and the PBs for substrate recognition are indicated. PLK5 has lost its catalytic activity in humans and expresses only a small portion of the kinase domain along with the PBD, the second PB has lost the conserved key residue involved in phosphosubstrate binding.
