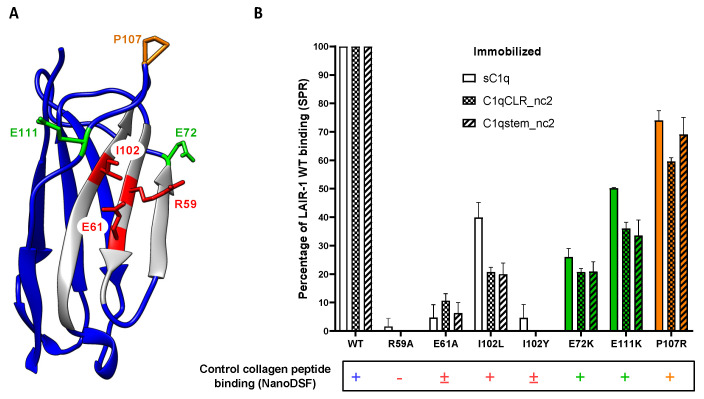
Figure 7.
Mutational analysis of LAIR-1 interaction with C1q. (A) Crystal structure of LAIR-1 Ig-like domain (PDB code: 3KGR). The side chains of the mutated residues are shown and labeled. The central binding residues are colored in red and the distal residues are shown in green and orange (as in Figure 5). (B) SPR measurements of the interaction of LAIR-1 WT and mutants with sC1q, C1qCLR_nc2 and C1qstem_nc2. Results are expressed as the percentage of the LAIR-1 WT response and error bars represent the SD of three different experiments. The detection of a binding signal of LAIR-1 variants with the positive collagen peptide control measured with the nanoDSF method is reported at the bottom. The presence or absence of the characteristic binding signal is reported with + or − symbols, respectively. The cases where a weak binding signal remains are indicated with a ± symbol. The raw data of the peptide interactions are presented in Figure 5B and Figure S5.