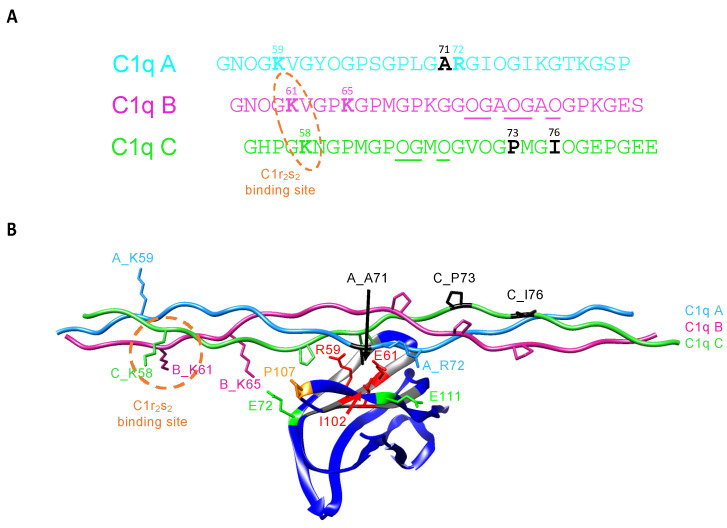
Figure 8.
Model of LAIR-1 Ig-like domain in complex with a C1q collagen stem. (A) Sequences of C1q A, B and C chains corresponding to the C1q collagen-like region shown in B. The three lysine residues constituting the C1r2s2 binding site (A_K59, B_K61 and C_K58) are in bold and the two essential lysines are surrounded with a dashed orange ellipse. The putative LAIR-1 binding OGXO motifs in this region are underlined. The remarkable residues are labeled with their position numbers. The three residues facing the LAIR-1 I102 in each alternative model are labeled in black. The “O” in the sequences stands for proline residues modified into hydroxyprolines, as determined in the literature [27,28]. (B) Model of LAIR-1 Ig-like domain (blue, PDB code: 3KGR) in interaction with the C1q CLR triple helix (A chain in cyan, B chain in pink and C chain in green). The collagen-binding groove of LAIR-1 is shown in grey and the side chains of the identified key binding residues are shown and labeled. Mutated lysine 65 and arginine 72 of C1q B and A chains, respectively, are labeled. The residues facing LAIR-1 I102 in each model are colored in black. The side chains of the identified OGXO motifs are shown in lines.