Table 1.
Solid-phase synthesis strategies for the azole-based peptidomimetics.
| No | Intermediate Resin | Cyclized Product | Key Strategies |
|---|---|---|---|
| 1 |
|

|
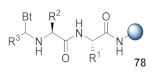
1. Formation of key intermediate 78 from resin-bound dipeptide, aldehyde, and benzotriazole; 2. Cyclization achieved via BF3∙Et2O treatment [49]. |
| 2 |

|

|
1. Formation of thiourea intermediate 80 at the N-terminal of the peptide; 2. Dehydrothiolation of 80 via Mukaiyama’s reagent [50]. |
| 3 |

|

|
1. Incorporation of secondary amino acid into the peptide sequence; 2. Cyclization via HgCl2, or DIC, or Mukaiyama’s reagent [51]. |
| 4 |

|

|
1. Oxidation and dehydrative cyclization of the Thr, Cys, Ser, diaminopropionic acid-containing dipeptides [52]. |
| 5 |
|

|
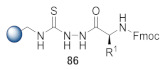
1. Backbone amide linker (BAL) strategy; 2. Thiosemicarbazide resin coupled with an amino acid to give thiourea intermediate 86; 3. Desulfurative cyclization of 86 with p-TsCl and pyridine; 4. Incorporation of 3-nitrobenzoyl functionality [53]. |
| 6 |

|
|
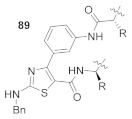
1. BAL strategy; 2. N-Bn-protected thiourea resin intermediate 88 was used to prevent undesired byproduct; 3. Dehydrative cyclization of 88 with 2-bromo-(3-nitrophenyl)ethenone [54]. |
| 7 |
|

|
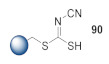
1. Traceless linker strategy; 2. Cyclization of 90 with ethyl bromoacetate to yield 1,3-thiazole [55]. |
| 8 |

|

|
1. Cyclization of 92 with hydrazides in the presence of Hg(Ac)2 [56]. |
| 9 |

|

|
1. One-pot Ugi-azide-4CR strategy [57]. |
