Abstract
Background
Sleep disturbances are challenging symptoms associated with mild cognitive impairment or dementia (MCIoD). This study assessed the feasibility of sleep monitoring and non-pharmacological interventions to improve the sleep of New Zealanders with MCIoD and their family carers.
Methods
A 5-week multi-modal intervention consisting of timed bright light therapy, physical activity, and sleep education was piloted. Sleep was monitored for a week at baseline and conclusion of the trial using actigraphy, diaries, and questionnaires alongside additional health and wellbeing information concerning both care recipients and carers.
Results
Fifteen pairs participated, 9 completed the trial. Patterns of attrition and participant feedback are discussed. Case studies showed that six of the care recipients had minor improvements to sleep efficiency. Some also had improved subjective sleep ratings and quality of life. Changes did not clearly translate to family carers. However, five of them also showed some improvements in sleep status and mental health. Health deterioration of care recipients may mask the effects of the intervention.
Conclusions
It is feasible to use non-pharmacological sleep interventions for people with MCIoD and their family carers. Given the limited treatment options, further consideration of such interventions in future research and clinical practice is warranted.
Trial registration
As this study was to assess the feasibility of proposed methods, it was an observational study without case-control groups nor a medical-based intervention, clinical registration was not required. A future full version of the trial would be registered with the Australian New Zealand Clinical Trails Registry.
Keywords: Actigraphy, Ageing, Caregiving, Dementia, Sleep problems, Non-pharmacological interventions
Key messages regarding feasibility
It was uncertain whether participants could be recruited and retained over the trial period in the community as opposed to an institutionalised setting. It was also uncertain whether the intervention and methods of assessment would be acceptable to people with dementia and their carers living in the community.
Participants rated the study methods and interventions as feasible and easy to use accept the sleep diary which, for some, was reported as onerous. Compliance was greater for the timed light aspect on the intervention rather than the physical activity. Participant retention was impacted by degrees of sleep problems and cognitive impairment as well as admittance into hospital or care facilities.
Recommendations are made around tailoring a trial towards those most in need (i.e. poorer sleep and limited access to bright light and physical activity) and using a person-centred approach to engage and deliver such intervention. Also, that actigraphy monitoring may be more acceptable and reliable without concurrent sleep diaries.
Introduction
Disordered, irregular sleep, and sleep deprivation have been associated with poorer cognition, physical and mental health, and mortality [1, 2]. Sleep deterioration, with ageing, has been related to multiple physiological, psychological, and health factors [3, 4]. As life expectancy rises, cases of mild cognitive impairment and dementia are increasing [5]. Sleep disturbances often occur in the early stages of cognitive decline and have been identified as among the most challenging symptoms associated with progressive dementias (such as Alzheimer’s disease) [6, 7]. These disturbances have been related to reduced activity and function of the circadian master clock in the hypothalamus, as well as psycho-social and behavioural factors [6]. Not surprisingly, the sleep of family carers is also often affected [8]. Situations where a person with mild cognitive impairment or dementia (MCIoD) and their family carer are both sleeping poorly tend to be those which are more difficult to manage [8, 9].
Pharmacological options for the symptoms of MCIoD (including sleep disturbances) often have side effects and limited effectiveness [10]. There is great emphasis on exploring non-pharmacological strategies to modify and manage the symptoms. Better management of sleep health could lead to improvements in waking symptoms and wellbeing for people with MCIoD as well as family carers. Interventions using timed bright light and/or physical activity can help stimulate the circadian system whilst promoting overall sleep health [11, 12]. Previous studies have found that interventions combining bright light therapy with physical or social activities as well as sleep hygiene education can promote more robust sleep timing and less disturbed sleep among people with dementia [11–14]. However, community-based trials are lacking and the feasibility of conducting such research in New Zealand (NZ) has yet to be explored. The NZ context differs from previous studies with regards to population size, cultural diversity, as well as sleep and age-related healthcare services. Action plans focus on supporting families affected by dementia to live their best possible lives [15]. Often, there is desire to remain at home under the support of family; this is particularly the case among older Māori (the indigenous people of NZ) [16]. Research to understand and meet such objectives are growing but, to date, sleep health is yet to be addressed. Pilot data are required before designing and implementing further trials among NZ’s diverse populations.
Aims and objectives
To pilot a community-based approach for gathering objective and subjective data on the sleep of people with MCIoD and their family carer in NZ.
To design and examine the feasibility of a trial for testing non-pharmacological interventions to improve sleep and waking function.
Feasibility outcomes included the acceptability of the protocol and usability of measures as well as positive impacts of the intervention. These were measured by assessing the quality of data, changes across the intervention, as well as gathering feedback both during weekly phone conversations and using formal feedback questionnaires post trial. Success of feasibility was gauged on a case-by-case basis by subjective responses as well as the objective sleep and wellbeing measures. Feedback was used to identifying the most useful measures, appropriate outcome variables, and factors affecting participant compliance and withdrawal (see “Data analysis” section for more details).
Methods
Participants
Fifteen pairs including a person with MCIoD (“care recipients”) and their family carers (“carers”) were recruited via Alzheimers Wellington (a local organisation offering support and advocacy services for families affected by dementia) as well as via community advertisements, presentations, and referrals from healthcare professionals. Due to the pilot nature of the study, convenience sampling was used. Inclusion criteria were living together in the Wellington community, the care recipient being aged ≥ 65 years, and that they and/or their carer had an interest in improving their sleep. Due to the underdiagnoses of dementia [5], status of MCIoD was based on participants’ report and involvement with dementia-related services. Exclusion criteria were having eye conditions or taking medications which could cause photosensitivity to the light therapy element of the protocol [17].
Written consent was gathered from each pair. Verbal assent was also recorded during the study briefing, to offer protection and/or an alternative for those with reduced cognitive capacity. Participant’s doctors were informed and given the opportunity to notify the researcher of any further contraindications for bright light exposure or contact the research team with any concerns.
Materials
Sleep/wake activity of the pairs was measured for 7 days at baseline (Time 1) and during the fifth week of the intervention (Time 2) using the Actiwatch-2 (Mini Mitter, Philips, Respironics) worn on the non-dominant wrist. The Actiwatch-2 has a button serving as an event marker for participants to press at relevant times (e.g. at bedtime), and a sensor for measuring the amount and duration of ambient white light exposure in units of lux (from the wrist). Activity counts were recorded in 1-min intervals and the records were analysed using custom software provided by the manufacturer, using the medium sensitivity threshold. Time in bed was defined via the event marker and light data from the Actiwatch-2 and a sleep diary in the form of 24-h timelines. Participants recorded the times of sleep start and end for any sleep that was 10 min or longer. Diaries for the care recipients were maintained throughout the intervention to collect information on compliance.
Questionnaires were completed by the pairs at Times 1 and 2. These included demographic information as well as validated questionnaires concerning sleep [18, 19], health [20, 21], symptoms, and quality of life of those with CIoD [22–24] and the impact of care [25] (see Table 1). Open-ended questions also prompted feedback on the protocol and ratings of each aspect of the methods and intervention. This information was evaluated regardless of whether a pair completed the entire protocol.
Table 1.
Content of questionnaires completed by care recipients and carers
| Completed by: | |||
|---|---|---|---|
| Measure | Reference | Care recipient | Carer |
| Demographics | Age, gender, type of diagnosis, caregiving timing, and relief | ✓ | ✓ |
| Sleep timing and quality | Pittsburgh Sleep Quality Index (PSQI) range = 0–21, > 5 = “disturbed sleep”] [18] | ✓ | ✓ |
| Dementia-related sleep disturbance | Sleep Disorders Inventory (SDI) range = 0–12/”severely disturbed sleep” [19] | ✓ | |
| Health | Self-Administered Comorbidity Questionnaire (SCQ [20]) and medication list | ✓ | ✓ |
| Cognitive impairment | Mini-Mental State Examination (MMSE) range = 30–0, > 23 = “no impairment,” 19–23 = “mild cognitive impairment,” 10–18 = “moderate cognitive impairment,” and ≤ 9 = “severe cognitive impairment” [22] | ✓ | |
| Dementia-related symptoms | Revised Memory and Behaviour Problems Checklist (RMBPC) global score (0–96) plus sub-scores for memory (range = 0–28), depression (range = 0–36), and disruptive behaviours (range 0–32) with higher scores indicating increased frequency of behaviours and negative carer affect [23] | ✓ | |
| Quality of life of recipient | Quality of Life in Alzheimer’s Disease: Patient and Caregiver Report (QOL-AD) range = 52 (excellent)—13 (poor) [24] | ✓ | ✓ |
| Mental health | Hospital Anxiety and Depression Scale (HAD), range 0–21, 0–7 = “normal,” 8–10 = “borderline,” 11–21 = heightened risk [21] | ✓ | |
| Impact of caregiving | Carers of Older People in Europe index (COPE) range for positive impact of caring items = 15–0(< 12 = reduced coping). Range for negative impact of caring items = 0–18 (> 12 = reduced coping) [25] | ✓ | |
Procedure
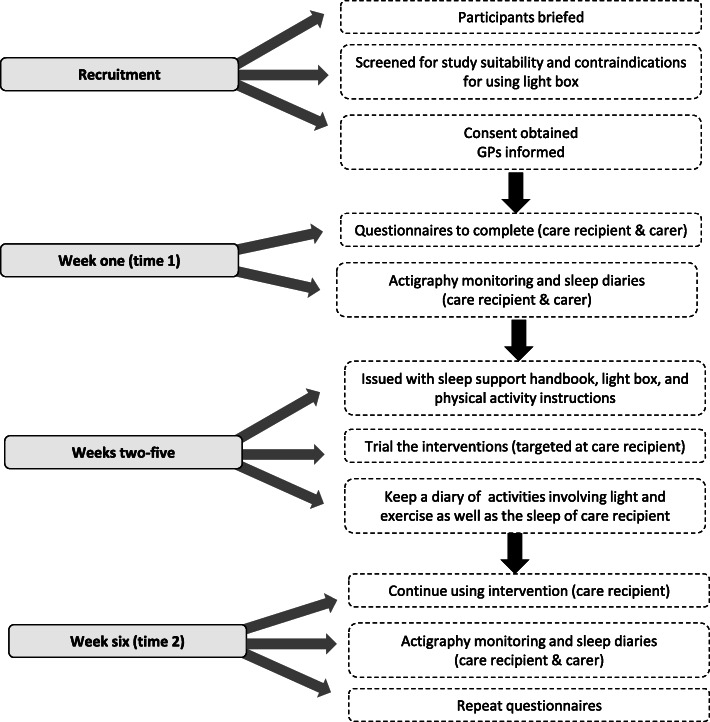
Figure 1 provides an overview of study procedures. Questionnaires were completed by the care recipient in the presence of the researcher and carer, to support completion. In cases when they were not cognitively able, the carer acted as a proxy. The interventions included timed bright light therapy, exercise, and sleep hygiene education. A day-light light box was provided (Uplift Technologies Inc., 2009) as an alternative to natural light. This provides up to 10,000 lux of illumination at a comfortable sitting distance (30–33 cm) [17]. The proposed exercise regime was 30–40 min of walking or yoga style stretches and movement (participants were loaned a senior’s exercise DVD and were given information about safe home exercise). A ‘Sleep Support Handbook’ was developed for the study that contained information on sleep with ageing and dementia, and sleep hygiene guidelines.
Fig. 1.
Overview of the study protocol
During the 5-week trial, care recipients were encouraged to get 30 min of bright light exposure every morning (or on as many mornings as possible) between 9 and 11 am, using either the light box or natural light. They were also asked to undertake the low-intensity exercises on as many days as possible during the middle of the day (11am–2pm). Carers were encouraged to join in with physical activities or supervise as they saw necessary. Finally, participants were encouraged to try some of the ideas/solutions offered in the handbook, and to note in the sleep diaries instances when they had tried new things. Participants were telephoned by the researcher on a weekly basis to remind them of the study protocol, gauge feasibility outcomes, and answer any questions regarding the study. They also had access to a free phone number to contact the researcher throughout for advice or assistance.
Data analysis
Actigraphy records were analysed with the diary data to identify rest periods. Fifty percent of records were double-scored and anomalous responses and outliers were checked (average agreement rate = 84.8% for the care recipient records, 92.1% for carers). Sections of complete inactivity (due to the actiwatch not being worn or for unknown reasons) were excluded. Rest periods were categorised into night and day. Night was defined as the time the participant initially went to bed intending to sleep (bedtime) until the final morning rising (rise time). Standard actigraphy variables were derived from the software (e.g. total sleep time, sleep efficiency, number of awakenings) and compared for each participant between Times 1 and 2. Global scores were calculated for each validated questionnaire and its subscales. For scales with established threshold values, participants were categorised according to whether they scored above or below the thresholds. The Pittsburgh Sleep Quality Index (PSQI) was key, with scores above 5 indicating problematic sleep [18].
Compliance with the intervention was estimated from the diaries (calculated from the number of days with light or activity logged within and outside of the requested time frames) as well as by comparing Time 1 light (9am–11am) and activity data (11am–2pm) from the Actiwatch 2’s to Time 2 data. Descriptive statistics from the nominal questions in the feedback form were calculated and field notes from home visits and telephone conversations collated. Due to the small and heterogeneous sample, the effect and feasibility of the protocol and intervention was based on case studies [26]. Individual scores were plotted for within-subject comparisons between Times 1 and 2, and to visually compare the distribution of results between participants who completed the trial versus those who did not.
Results
Fifteen pairs took part. The demographic details of the care recipients and their study outcome are in Table 2. All but two pairs were spouses (opposite-gender couples). Pairs 6 and 14 were parent and child. Due to the small convenience sample, ethnicity was not recorded. The majority (n = 11) of the carers were not in paid employment. However, four were also working 20–40 h per week (ID’s 5, 6, 8, and 14). Summary data for key questionnaire and actigraphic data are provided in Table 3. This shows that care recipients had poorer and more variable objective sleep duration and efficiency compared to carers. In contrast, care recipients’ PSQI sores generally indicated less subjective sleep disturbance than carers.
Table 2.
Brief demographic details with outcome summaries
| ID | Age | Gender | Diagnosis | MMSE | Carer age | Outcome summary |
|---|---|---|---|---|---|---|
| Those who completed: | ||||||
| 1 | 73 | Male | VD | 15 | 73 | Dementia-related deterioration, minor improvements to care recipient’s PSQI and actigraphic sleep |
| 2 | 82 | Male | VD | 13 | 74 | Dementia-related deterioration, PSQI improved for both |
| 3 | 72 | Male | AD | 14 | 71 | Good quality sleep at onset, minor improvement to care recipient’s PSQI |
| 4 | 76 | Male | Undefined | 23 | 74 | Good quality sleep at onset, no improvements observed, care recipient had marked daytime sleepiness |
| 5 | 82 | Male | LBD | 5 | 68 | Marked improvements to both subjective and actigraphic sleep variables as well as waking function of care recipient |
| 6 | 83 | Female | Undefined | 27 | 46 | Minor subjective and actigraphic improvements to sleep, however carer reported deterioration to care recipient’s sleep |
| 7 | 73 | Female | AD | 16 | 78 | Good quality sleep at onset, no improvements observed |
| 8 | 66 | Male | AD | 18 | 67 | Good sleep quality at onset, no improvements observed. Struggled with protocol |
| 9 | 80 | Male | VD | 7 | 54 | Minor improvements to actigraphic sleep of both. Carer reported stress and sleep disturbed by care recipient |
| Those who withdrew: | ||||||
| 10 | 80 | Female | AD | 21 | 81 | Withdrew due to care recipient moving into care home at end of week 1 |
| 11 | 72 | Male | AD | 13 | 68 | Withdrew as care recipient admitted to hospital |
| 12 | 88 | Male | MCI | 29 | 75 | Withdrew after losing motivation after period of respite care |
| 13 | 83 | Female | VD | 20 | 82 | Withdrew at the end of week 1, sleep not considered problematic enough |
| 14 | 89 | Female | Undefined | 23 | 55 | Withdrew as intervention protocol considered too stressful |
| 15 | 73 | Male | VD | 28 | 55 | Withdrew during week 1 due to care recipient moving into care facility after a fall |
Abbreviations: VD vascular dementia, AD Alzheimer’s disease, LBD Lewy body dementia, MCI mild cognitive impairment, undefined type of dementia unknown/not specified in diagnosis, MMSE Mini-Mental Status Exam, PSQI Pittsburgh Sleep Quality Index
Table 3.
Descriptive statistics and paired summaries of key variables for participants who started and completed the protocol (first and second line for each variable respectively)
| Time 1 | Time 2 | |||||
|---|---|---|---|---|---|---|
| Variable (range of scale) | N | Median | (range) | N | Median | (range) |
| Care recipient | ||||||
| Sleep duration night, hours | 15 | 8.0 | (5.5–10.6) | |||
| 9 | 8.1 | (6.7–9.8) | 9 | 8.2 | (5.7–12.3) | |
| Sleep efficiency night (%) | 15 | 85.0 | (61.3–98.4) | |||
| 9 | 85.0 | (62.0–95.9) | 9 | 85.4 | (61.4–96.5) | |
| Sleep duration day, minutes | 15 | 47.0 | (0.0–78.0) | |||
| 9 | 51.5 | (0.0–78.0) | 9 | 29.5 | (0.0–192.0) | |
| PSQI (0–21) | 15 | 4.0 | (1.0–13.0) | |||
| 9 | 4.0 | (1.0–8.0) | 9 | 3.0 | (2.0–7.0) | |
| SDI-AD (0–12) | 14 | 0.2 | (0.0–4.2) | |||
| 9 | 0.9 | (0.0–4.0) | 9 | 1.1 | (0.0–3.0) | |
| MMSE (30–0) | 14 | 18.0 | (7.0–29.0) | |||
| 9 | 15.0 | (5.0–27.0) | 9 | 15.0 | (6.0–26.0) | |
| RMBPC Behaviour frequency (0–32) | 15 | 3.5 | (0.0–11.0) | |||
| 9 | 3.0 | (0.0–11.0) | 5.0 | (2.0–10.0) | ||
| RMBPC Behaviour reaction (0–32) | 15 | 3.5 | (0.0–19.8) | |||
| 9 | 3.3 | (0.0–12.0) | 9 | 3.5 | (0.0–24.0) | |
| QOL-AD (CR rated), (52–13) | 14 | 39.0 | (24.0–48.0) | |||
| 8 | 39.5 | (24.0–45.0) | 9 | 36.0 | (18.0–49.0) | |
| QOL-AD (C rated), (52–13) | 15 | 30.0 | (23.0–40.0) | |||
| 9 | 30.0 | (23.0–40.0) | 9 | 34.0 | (22.0–40.0) | |
| Carer | ||||||
| Sleep duration night, hours | 14 | 7.5 | (5.9–8.9) | |||
| 8 | 7.9 | (6.1–8.9) | 7 | 7.6 | 6.7–9.1 | |
| Sleep efficiency (% sleep night) | 14 | 89.6 | (72.5–93.7) | |||
| 8 | 90.0 | (85.1–93.7) | 7 | 87.1 | (83.3–92.4) | |
| Sleep duration day, minutes | 14 | 7.0 | (0.0–80.0) | |||
| 8 | 0.0 | (0.0–80.0) | 7 | 0.0 | (0.0–47.5) | |
| PSQI (0–21) | 14 | 8.0 | (1.0–10.0) | |||
| 8 | 8.0 | (1.0–9.0) | 9 | 5.0 | (2.0–9.0) | |
| COPE positive (15–0) | 15 | 11.0 | (4.0–14.0) | 9 | 10.0 | (4.0–15.0) |
| 9 | 10.0 | (4.0–14.0) | ||||
| COPE negative (0–18) | 15 | 5.0 | (1.0–12.0) | 9 | 10.0 | (0.0–12.0) |
| 9 | 6.0 | (1.0–12.0) | ||||
| HADS-Anxiety (0–21) | 15 | 4.0 | (0.0–12.0) | 9 | 6.0 | (1.0–12.0) |
| 9 | 4.0 | (0.0–12.0) | ||||
| HADS-Depression (0–21) | 15 | 4.0 | (0.0–9.0) | 9 | 5.0 | (0.0–11.0) |
PSQI Pittsburgh Sleep Quality Index, SDI-AD Sleep Disorders Inventory Alzheimer’s Disease, MMSE Mini Mental Status Exam, RMBPC Revised Memory Behaviour Problem Checklist (global scores unavailable due to missing data), QOL-AD Quality of Life in Alzheimer’s disease” Patient and Caregiver report, COPE Carers of Older People in Europe index, HADS Hospital Anxiety and Depression Scale
Six of the pairs withdrew during the trial. Care recipients in these pairs tended to be less cognitively impaired (median MMSE = 20.5, range = 13.0–29.0) compared to those who completed the trial (median MMSE = 15.5, range = 7.0–27.0). However, the RMBPC scores indicated that those who withdrew tended to have more frequent and disruptive behaviours (median = 6.3, range = 0.0–10.5 vs. median = 3.0, range = 0–11). The carers reaction rating to these behaviours was also higher among those who withdrew (median = 5.8, range = 0–19.8) compared to those who completed the trial (median = 3.0, range = 0–12). Two withdrawal “types” were identified among the care recipients. Firstly, those who had less severe symptoms of dementia with minor sleep problems and lost interest or were confused by the study protocol (pairs 12, 13, and 14); and secondly, those who had more advanced symptoms of dementia with substantial sleep problems but were admitted to hospital or a care facility during the trial period and so could not complete the trial (pairs 10, 11, and 15). All pairs rated the actigraphy and questionnaires as ok-easy to use. Only one rated the diary as difficult, but some carers noted the challenge of being able to reliably document sleep and wake bouts across the 24-h day for care recipients who had very fragmented sleep. For those who completed the trial (n = 9 pairs), diary-reported compliance with the bright light therapy ranged from 37 to 97% of the days during the 5-week intervention (median 72%). Compliance with the exercises was lower (3–86% days, median 55%). The interventions were not always used in the specified timeframes (56% of the time and 38% of the time respectively). The Actiwatch 2 data showed an 85% increase in mean light exposure for care recipients within the 9am–11am timeframe between baseline and trial completion (mean T1 = 4905 lux, SD = 5377 lux vs. mean T2 = 9077, SD = 14,709) compared to just a 2% increase in physical activity counts within the 11am–2pm timeframe (mean activity counts T1 = 27,635, SD = 15,096 vs. mean activity counts T2 = 28,181, SD = 13,338). There was no clear pattern of baseline measures being reliable predictors for trial outcomes. Feedback data revealed that none of the pairs found the light box difficult to use. However, two found the exercise DVD difficult and one reported difficulty with the education booklet. Seven of the nine said that they would recommend the interventions to others (the others said they maybe would).
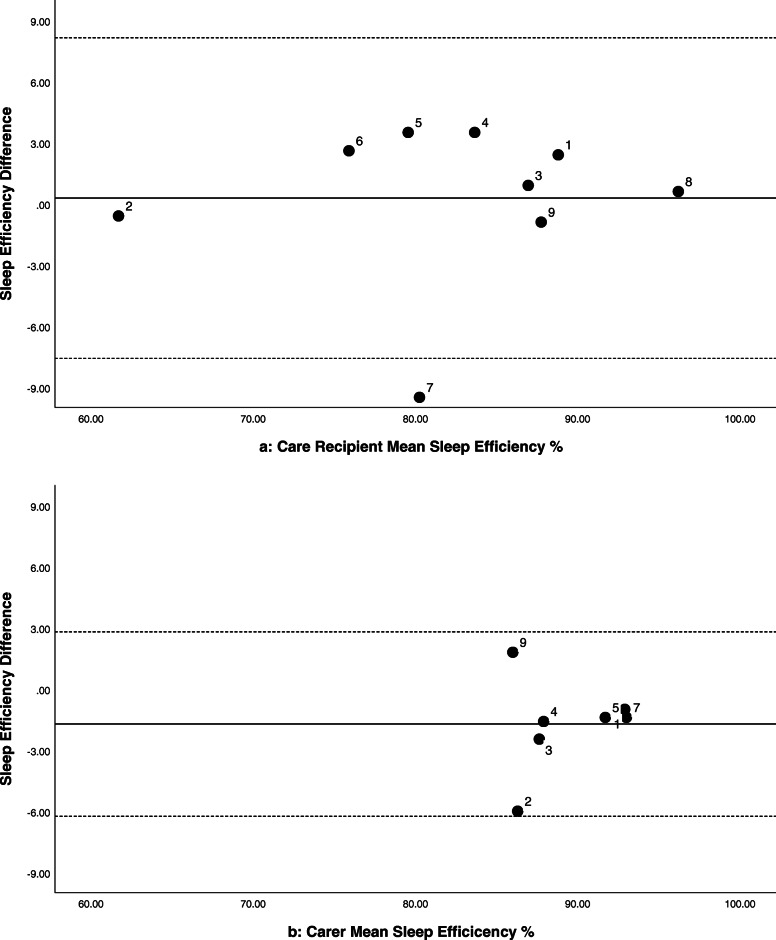
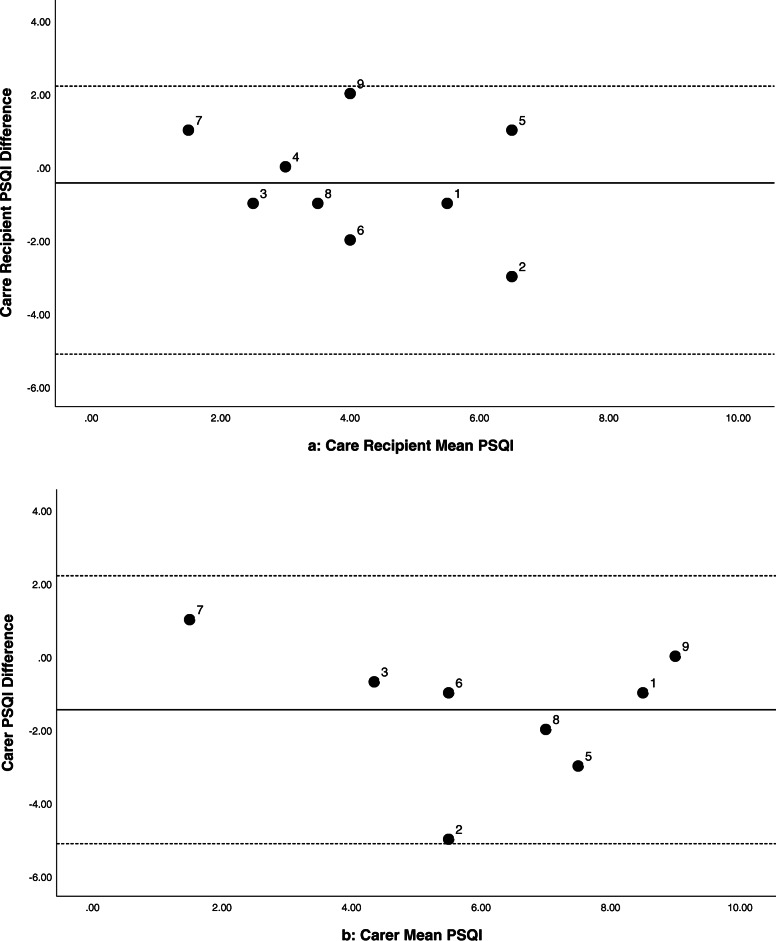
Six of the care recipients showed minor improvements in their sleep efficiency at night (0.6–3.6%, see Fig. 2a). Their median time in bed was 10.2 h (range = 8.7–11.9 h), indicating an increase of 4–22 min more sleep at Time 2. While the grouped data are variable, there were cases with more marked improvements in sleep and wellbeing (e.g. Participant 5, case study presented elsewhere) [26]. The majority (six) of the care recipients scored normal/good on the PSQI at Time 1 (< 5). Nevertheless, five showed 1–3 points of improvement on their PSQI scores at Time 2 (see Fig. 3a). Actigraphic and subjective improvements in sleep (PSQI or SDI scores) were not clearly related. Of the five care recipients who had improved PSQI ratings at Time 2, two also had improved scores on the MMSE (3–4 points) and three had improvements to their quality of life as rated by their carer QOL-AD (2–7 points). However, between Times 1 and 2, six had reductions of 1–7 points in their MMSE score. Furthermore, many of the behavioural symptoms associated with dementia (as indicated by participant feedback and scores on the RMBPC or QOL-AD) deteriorated across the trial period. Six out of the nine carers had PSQI scores that indicated disturbed sleep (> 5) at Time 1. Five had 1–5 points of improvement in their PSQI scores between Times 1 and 2 (see Fig. 3b). Of these five, three also had decreased depression scores at Time 2 (as indicated by the HADS). However, the majority (seven) scored within the normal range for symptoms of anxiety and depression at Time 1. Improvements to the care recipient’s sleep did not always translate into improved sleep, mood, or coping of their family carer (Figs. 2b and 3b).
Fig. 2.
Bland-Altman plots comparing sleep efficiency at night between Times 1 and 2 for care recipients 2(a) and family carers 2(b)
Fig. 3.
Bland-Altman plots comparing PSQI scores between Times 1 and 2 for care recipients (3a) and family carers (3b)
Discussion
This study indicates that a combined intervention using bright light therapy and/or therapeutic exercise with sleep hygiene education is feasible for people with MCIoD and their family carers at home. Given the paucity of options for treating dementia, the intervention has potential for improving the sleep and wellbeing of older people at risk or diagnosed with dementia. The mixed-methods approach and use of case studies including both care recipients and care partners was novel, allowing investigation into the context of conducting such a study in the NZ community. The protocol was considered feasible and suggestions for future trials were obtained. The small heterogeneous sample limited the ability to measure the clinical effectiveness of the interventions, or to determine the characteristics of those most likely to benefit. However, some pairs did report benefits to their sleep and waking wellbeing. In other cases, a rapid deterioration of health masked any possible improvements or prevented participants from being able to complete the trial. These mixed findings are concordant with previous research with people living in institutions or the community, using both cross-sectional studies and randomised controlled trials [27]. They are representative of the clinical reality of families affected by dementia.
The interventions offer a low-risk, drug-free option for managing dementia-related sleep disturbances. They were designed to address well-defined physiological and psychosocial processes affecting sleep and waking function (most notably stabilising the function of the circadian master clock) [6, 7]. The protocol in the present study differed from previous community-based trials (e.g. 13, 28) in that bright light was recommended at mid-morning, using a higher intensity and for a shorter duration. The exercise component also had a recommended time frame. Delivering the interventions this way was considered more suitable for the participants, since dementia-related symptoms and behaviours often exacerbate towards evening, which is also a time of high care demands [6]. The timing of the light and exercise exposures was also anticipated to minimise the likelihood of the interventions having an arousing effect on the care recipient before bedtime [11, 12].
Compared to previous community-based studies [13, 28], the present sample of care recipients had more varied types of dementia-related impairment. They also had less problematic sleep at baseline, as indicated by actigraphic and subjective reports as well as higher baseline light exposure (more than 2000 lux). Similarly, the sample of carers had relatively good actigraphic sleep compared to those in previous samples. However, their subjective ratings indicated a high prevalence of sleep problems which was more comparable [8]. Discrepancies between subjective and objective sleep data of carers is not unusual and likely associated with the psychosocial impact of caring on the symptoms of insomnia [8, 29]. Carers in the present sample appeared to have reduced likelihood of anxiety or depression compared to previous samples of NZ carers [25] which may have contributed to different sleep outcomes.
Variable improvement in sleep problems associated with MCIoD may be attributable to a range of factors. These include variability in comorbid diseases and demographic factors, the fluctuating nature of sleep and dementia-related behaviours, as well as response bias, possible placebo effects, and the impact of researcher support. Comparisons between studies are also confounded by differences in sleep measures and intervention characteristics. The course of cognitive deterioration and factors necessary for stable sleep timing and efficiency (including damage to the eye, pineal gland, and circadian system as well as presence of another primary sleep disorder) are also expected to affect intervention effectiveness [27].
Based on this study, it is recommended that future trials are tailored towards care recipients who have limited access to bright light and physical activity, as well those with poor sleep status at baseline. A larger randomised controlled trial would enable greater monitoring of the impact of the intervention while controlling for biases. The Inclusion of qualitative elements in such trials is recommended to strengthen our understanding of the psychosocial elements of sleep health with ageing, MCI, and dementia as well as providing a person-centred perspective on the effectiveness of the interventions in this highly variable population.
Considering the variation between families affected by dementia as well as issues with recruitment, compliance, and attrition in the present study the findings presented here cannot be generalised to all families affected by MCIoD. With appreciation for such diversity and challenges, future trials may benefit from a more personally tailored approach to engagement and delivery. This may also reduce research load on the participants. This could be achieved through using co-designed research methods and customised interventions, automated rather than diary-assisted scoring of actigraphy data (removing the need for keeping dairies) [30], and delivering interventions via primary healthcare or pre-existing home care services with a support person to facilitate uptake. Research of this nature are now underway within a broad research team in NZ (including the first author). Supported by the Ageing Well National Science Challenge, “Ageing Well through Eating, Sleeping, Socialising and Mobility Programme (AWESSoM)” will assess and support key pillar of health non-pharmacologically within at-risk groups of older people living in New Zealand including older Māori, Pacific Islanders, and care home residents.
Conclusion
With a rapidly ageing population, increases in New Zealanders affected by dementia are projected. Many are supported at home informally via family. This model is becoming increasingly critical for regulating the costs to the healthcare system as well as for facilitating older New Zealanders to live well with advancing age and dementia [5]. This study indicates that a combined non-pharmaceutical intervention for sleep is feasible for people with MCIoD and their family carers at home, with limited research burden. While the effect of the intervention was variable at the group level, some individuals reported improvements to sleep and waking status. We conclude that, given the lack of options for treating dementia, the intervention has potential for improving the sleep and wellbeing of older people at risk or diagnosed with dementia and further trials are warranted.
Acknowledgements
We would like to acknowledge the participants of this study and Kanchana Pathirana for assistance with data entry. Funders of this research include the Health Research Council of New Zealand feasibility grant (number 11/562), and Maurice and Phyllis Paykel Trust (project and equipment grant, 2010).
Authors’ contributions
RG designed the study protocol and applied for funding, recruited participants, and collected data. RG also led the analyses and write up of the results. LJ contributed to the study with regard to engagement of people with dementia living in the community and interpretation of results. TD informed the study with regard to its application with clinical practice and community healthcare. PG oversaw the study, informing the study design and protocol with regard to their expertise in circadian health, applying for funding, and guiding interpretation of results. All authors read and approved the final manuscript.
Funding
Funders of this research include the Health Research Council of New Zealand feasibility grant (number 11/562), and Maurice and Phyllis Paykel Trust (project and equipment grant, 2010). Neither funding body had any role with regards to the design of the study or the collection, analysis, or interpretation of data nor in writing the manuscript.
Availability of data and materials
The protocol, study documents, and datasets produced and analysed during the current study are available from the corresponding author on reasonable request. In alignment with the Health and Disability Ethics Committee of NZ regulations, data are stored for 10 years in electronic and paper form in secure locations at the Sleep/Wake Research Centre and will be unavailable after July 2022.
Declarations
Ethics approval and consent to participate
This study was an observational study to assess the feasibility of proposed methods without case-control groups. The interventions assessed in the study were not of a medical nature (but rather recommendations concerning sleep and the timing of common behaviours). Therefore the New Zealand Central Health and Disability Ethics Committee (CEN/11/01) did not require clinical registration to approve the study processes. A future full version of the trial would be registered with the Australian New Zealand Clinical Trails Registry. Written consent was gathered from each pair by the lead researcher (RG). This included reading and agreeing to the information sheet regarding reporting and publication of anonymised data. Verbal assent was also recorded during the study briefing.
Consent for publication
Consent for publication was received via signed consent forms where participants agreed that they have read an understood the information sheet including information about anonymous use of data within publications.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rosemary Gibson, Email: r.gibson@massey.ac.nz.
Anthony Dowell, Email: tony.dowell@otago.ac.nz.
Linda Jones, Email: l.m.jones@massey.ac.nz.
Philippa Gander, Email: p.gander@massey.ac.nz.
References
- 1.Adams RJ, Appleton SL, Taylor AW, Gill TK, Lang C, McEvoy RD, et al. Sleep health of Australian adults in 2016: results of the 2016 Sleep Health Foundation national survey. Sleep Health. 2017;3(1):35–42. 10.1016/j.sleh.2016.11.005. [DOI] [PubMed]
- 2.Da Silva AA, De Mello RGB, Schaan CW, et al. Sleep duration and mortality in the elderly: A Systematic review with meta-analysis. BMJ Open. 2016;6(2):e008119. doi: 10.1136/bmjopen-2015-008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 4.Scullin MK, Bliwise DL. Sleep, Cognition, and normal aging: integrating a half century of multidisciplinary research. Perspectives on Psychological Science. 2015;10(1):97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Incorporated ANZ. Dementia economic impact report 2016. 2017. [Google Scholar]
- 6.Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment. Drugs. 2001;15:777–796. doi: 10.2165/00023210-200115100-00004. [DOI] [PubMed] [Google Scholar]
- 7.Saeed Y, Abbott SM. Circadian disruption associated with Alzheimer’s disease. Curr Neurol Neurosci Rep. 2017;17(4):29. doi: 10.1007/s11910-017-0745-y. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, RH, Gander, PH. Factors associated with the sleep of carers: a survey of new Zealanders supporting a family member with cognitive impairment or dementia. Dementia. 2020; online ahead of print doi:10.1177/1471301220915071 [DOI] [PubMed]
- 9.Gibson RH, Gander PH, Jones LM. Understanding the sleep problems of people with dementia and their family caregivers. Dementia. 2014;13:348–363. doi: 10.1177/1471301212473884. [DOI] [PubMed] [Google Scholar]
- 10.McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev. 2016;11:CD009178. doi: 10.1002/14651858.CD009178.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buxton OM, Lee CW, L'Hermite-Balériaux M, Turek FW, van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 12.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCurry SM, Pike KC, Vitiello MV, et al. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: Results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59(8):1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. J Gerontol A Biol Sci Med Sci. 2007;62(1):67–72. doi: 10.1093/gerona/62.1.67. [DOI] [PubMed] [Google Scholar]
- 15.New Zealand Dementia Cooperative, Alzheimers New Zealand, Dementia New Zealand. Improving Dementia Services in New Zealand. Dementia Action Plan 2020-2025. Available from: https://dementia.nz/wp-content/uploads/2020/06/New-Zealand-Dementia-Action-Plan.pdf.
- 16.Dyall L. Dementia: Continuation of health and ethnic inequalities in New Zealand. NZ Med J. 2014;127:68–80. [PubMed] [Google Scholar]
- 17.Wirz-Justice A, Benedetti F, Terman M. Chronotherapies for affective disorders: a clincian's manual for light and wake therapy. Basel, Switzerland: Karger; 2008. [Google Scholar]
- 18.Buysse DJ, Reynolds ICF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Tractenberg RE, Singer CM, Cummings JL, Thal LJ. The sleep disorders inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s disease. J Sleep Res. 2003;12(4):331–337. doi: 10.1046/j.0962-1105.2003.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatra Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. 'Mini mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral-problems in dementia - the revised memory and behavior problems checklist. Psychol Aging. 1992;7(4):622–631. doi: 10.1037/0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 24.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Roud H, Keeling S, Sainsbury R. Using the COPE assessment tool with informal carers of people with dementia in New Zealand. NZ Med J. 2006;119(1237):U2053. [PubMed] [Google Scholar]
- 26.Gibson RH, Gander PH, Jones LM, Dowell AC. Non-pharmacological interventions for managing dementia-related sleep problems within community dwelling pairs: a mixed-method approach. Dementia. 2016;16:967–984. doi: 10.1177/1471301215625821. [DOI] [PubMed] [Google Scholar]
- 27.Brown CA, Berry R, Tan MC, Khoshia A, Turlapati L, Swedlove F. A critique of the evidence base for non-pharmacological sleep interventions for persons with dementia. Dementia. 2013;12(2):210–237. doi: 10.1177/1471301211426909. [DOI] [PubMed] [Google Scholar]
- 28.Friedman L, Spira AP, Hernandez B, et al. Brief morning light treatment for sleep/wake disturbances in older memory-impaired individuals and their caregivers. Sleep Med. 2012;13:546–9. [DOI] [PMC free article] [PubMed]
- 29.Rowe MA, McCrae CS, Campbell JM, et al. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. J Clin Sleep Med. 2008;4:362–9. [PMC free article] [PubMed]
- 30.Gibson RH, Gander PH.. Monitoring the sleep patterns of people with dementia and their family carers in the community. Australas J Ageing. 2019;38:47–51. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol, study documents, and datasets produced and analysed during the current study are available from the corresponding author on reasonable request. In alignment with the Health and Disability Ethics Committee of NZ regulations, data are stored for 10 years in electronic and paper form in secure locations at the Sleep/Wake Research Centre and will be unavailable after July 2022.