Abstract
Obesity is a major worldwide health problem that is related to most chronic diseases, including male infertility. Owing to its wide impact on health, mechanisms underlying obesity-related infertility remain unknown. In this study, we report that mice fed a high-fat diet (HFD) for over 2 months showed reduced fertility rates and increased germ cell apoptosis, seminiferous tubule degeneration, and decreased intratesticular estradiol (E2) and E2-to-testosterone ratio. Interestingly, we also detected a decrease in testicular fatty acid levels, behenic acid (C22:0), and docosahexaenoic acid (DHA, 22:6n-3), which may be related to the production of dysfunctional spermatozoa. Overall, we did not detect any changes in the frequency of seminiferous tubule stages, sperm count, or rate of in vitro capacitation. However, there was an increase in spontaneous and progesterone-induced acrosomal exocytosis (acrosome reaction) in spermatozoa from HFD-fed mice. These data suggest that a decrease in E2 and fatty acid levels influences spermatogenesis and some steps of acrosome biogenesis that will have consequences for fertilization. Thus, our results add new evidence about the adverse effect of obesity in male reproduction and suggest that the acrosomal reaction can also be affected under this condition.
Keywords: cholesterol, estradiol, fat acid, testis, testosterone
INTRODUCTION
Infertility is a condition that affects approximately 15% of couples worldwide; almost half of the cases involve male-related factors.1 Among the risk factors of male infertility, one of the most frequent is obesity, which is a chronic condition present in 12% of men throughout the world.2,3 This notion is sustained by research showing that reversing obesity (weight loss) by lifestyle modifications or bariatric surgery allows one to recover some aspects of fertility.4,5 Studies in humans agree that, despite the absence of changes in luteinizing hormone (LH) or follicle-stimulating hormone (FSH) levels, a decrease in total testosterone and increases in estradiol (E2) and sex hormone-binding globulin (SHBG) are consistently detected in the serum of most obese infertile male patients.6 In addition, it seems that obesity is not related to sperm parameters such as viability, DNA damage, or changes in seminal parameters such as sperm concentration or motility.6,7,8 On the contrary, other studies have highlighted a longer time to conception; increased rate of embryonic abnormalities after in vitro fertilization; decreased sperm viability, concentration, and motility; and an increase in sperm DNA damage.9,10,11,12,13,14,15 On the other hand, reports on rats, mice, and rabbits have shown that diet-induced obesity impacts testicular physiology by affecting steroidogenesis,16,17,18,19,20 promoting disruption in the blood–testis barrier,16 increasing germ cell apoptosis,16,20,21 and decreasing total sperm count, concentration, and motility.16,17,19,21,22,23 In addition, an increase in abnormal sperm morphology reportedly leads to an increase in oxidative stress and DNA damage in spermatozoa.19,21,23,24
Cholesterol is a major structural component of the cell membrane; it also participates in diverse signaling pathways.25,26 In the testis, cholesterol is implicated in the fate of germ cells and is also a precursor of steroid hormones such as testosterone and E2. Once ejaculated, mammalian spermatozoa must activate a series of biochemical pathways that, as a whole, are known as capacitation.27,28 These processes allow them to experience an exocytotic event known as the acrosomal reaction (AR), which is required for them to fertilize the egg, and it is a good predictor of in vitro fertilization.28,29 Cholesterol efflux is a key event during mammalian sperm capacitation; blocking or hastening this process may prevent or quicken capacitation and the AR.30,31,32 Changes in lipogenesis in obese infertile patients are reflected in an increase in cholesterol and impairment of the AR.33 More recently, changes in fatty acid composition in spermatozoa have been detected in obese infertile men and are associated with low sperm quality.34
During spermatogenesis, Sertoli cells, as well as germ cells, convert dietary fatty acids into essential long-chain polyunsaturated fatty acids (PUFAs), including arachidonic acid (AA; 20:4n-6), eicosapentaenoic acid (C20:5n-3), docosapentaenoic acid (DPA; 22:5n-6), and docosahexaenoic acid (DHA; C22:6n-3), by alternating steps of elongation, desaturation, and β-oxidation.35,36,37 AA and DHA are considered essential fatty acids (EFAs) owing to their role during testis development and spermatogenesis. AA modulates mitochondrial function and mediates inflammation and apoptosis.38,39 DHA is the most abundant fatty acid in the testis and an integral component of the sperm membrane. In human spermatozoa, its levels are correlated positively with total sperm count, concentration, vitality, and progressive sperm motility, but negatively with the DNA fragmentation index.38 Cholesterol homeostasis and fatty acid biosynthesis are highly regulated processes, and abnormal levels of cholesterol or deregulation of fatty acid biosynthesis during a high-fat diet (HFD) intake could have serious cellular consequences in germ cells and spermatozoa and may lead to diseases such as infertility. Therefore, using an obesogenic model of HFD intake in mice, we investigated testicular damage, intratesticular hormone content, and cholesterol and fatty acid deregulation in the testis and their implications in sperm physiology (capacitation and AR).
MATERIALS AND METHODS
Animals and diet administration
All investigations were conducted in accordance with the rules established by the Consortium for Developing a Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching and by the National Research Council. All animal protocols were endorsed by the Chilean National Fund of Science and Technology (FONDECYT).
Twenty-one-day-old C57BL/6J male mice (just weaned) were randomly assigned to either the control (chow diet) group (Prolab RMN 3000, LabDiet, St. Louis, MO, USA) or HFD group (Dio Rodent Purified diet with 60% energy from fat, LabDiet). Food and water were given ad libitum, and the animals were housed four individuals per cage, under a cycle of 12 h light:12 h dark and controlled temperature at 22°C ± 1°C. No littermates were in the same experimental group (cage). A comparative analysis of experimental diets is shown in Supplementary Table 1. All animals were weighed early in the morning on the same day once a week to determine their weight gain. The end point of dietary treatment was at 90 days old (the duration of dietary treatment for these animals was 10 weeks).
Supplementary Table 1.
Comparative analyses of the experimental diets
| Nutrients | Chow diet (control) | HFD |
|---|---|---|
| Carbohydrates (%) | 32.13 | 25.9 |
| Protein (%) | 22.50 | 23.10 |
| Fat (%) | 12.90 | 34.90 |
| Cholesterol (ppm) | 199.00 | 301.00 |
| Linoleic acid (%) | 1.63 | 4.70 |
| Linolenic acid (%) | 0.19 | 0.39 |
| Arachidonic acid (%) | 0.02 | 0.06 |
| Omega-3 fatty acids (%) | 0.37 | 0.39 |
| Total saturated fatty acids, (%) | 1.71 | 13.68 |
| Total monounsaturated fatty acids (%) | 1.80 | 14.00 |
| Energy (Kcal/g) | 4.20 | 5.10 |
| Calories provided by | ||
| Carbohydrates (%) | 59.12 | 20.30 |
| Protein (%) | 25.96 | 18.1 |
| Fat (ether extract) (%) | 14.93 | 61.60 |
HFD: high-fat diet
Glucose tolerance test
The glucose tolerance test was performed on 90-day-old males; blood glucose was determined using the Accu-Chek Active kit (Roche Diagnostics, Mannheim, Germany). Briefly, after a 6-h fast, an intraperitoneal injection of glucose (2 g kg−1 body weight [BW]) was administered to each male, and blood samples from the tip of the tail were collected after 30 min, 60 min, 90 min, and 120 min, according to Andrikopoulos et al.40
Evaluation of testicular damage and germ cell apoptosis
All 90-day-old animals were killed by cervical dislocation, and their body, testes, and epididymides were weighed. Subsequently, one testis was fixed in Bouin's solution, embedded in paraffin, and cut into 7-μm sections. These were mounted on slides and analyzed by periodic acid–Schiff (PAS) staining and counterstained with hematoxylin. The sections were examined under an Olympus CX31 microscope (Olympus, Tokyo, Japan). Pictures were taken using an Olympus 5XC-3 digital camera (Olympus), and morphometric analyses were performed by using ImageJ software (NIH, Bethesda, MD, USA). Representative alterations of seminiferous tubule structure, such as the presence of germ cells exfoliated toward the lumen, the absence of a lumen, and the number of seminiferous tubules with vacuoles,41 were quantified in a minimum of 100 randomly selected seminiferous tubules and in a minimum of three males per group. Germ cell death was first determined by microscopy through the presence of pyknotic cells and then corroborated by immunohistochemistry with an anti-caspase-3 antibody. The frequency of seminiferous stages was classified as described previously.42 All analyses were on a minimum of 100 seminiferous tubules randomly selected per each sample in four animals per group.
Immunohistochemistry
The active form of caspase 3 was detected by immunohistochemistry as previously described.43 Briefly, cross-sections of paraffin-embedded mouse testes, previously fixed in Bouin's solution, were subjected to antigen retrieval with 0.01 mol l−1 sodium citrate (pH 6.0) and heated until boiling. The samples were treated with 3% (v/v) H2O2 for 10 min, followed by a standard protein block reagent (Ultra V block, Freemont, CA, USA) applied for 10 min to prevent nonspecific binding. The slides were incubated overnight at 4°C with 1 mg ml−1 of anti-caspase-3 antibody (catalog no. #9661, Cell Signaling, Danvers, MA, USA) in phosphate-buffered saline (PBS, pH 7.4). After a washing, the sections were incubated with a biotinylated anti-rabbit secondary antibody (KPL, Gaithersburg, MD, USA) followed by streptavidin–biotinylated–peroxidase complex, an amplification reagent (biotinyl tyramide), and peroxidase-conjugated streptavidin (30 min each; EZ-Link Sulfo-NHS-SS-Biotin and NeutrAvidin Agarose Resins; Thermo Scientific, Fremont, CA, USA). Between each step, the slides were washed three times for 5 min with Tris-HCl buffer (pH 7.6) with 0.3 mol l−1 NaCl and 0.1% (v/v) Tween-20. Finally, a substrate–chromogen solution (0.8% [v/v] H2O2[substrate] and 3,3'-diaminobenzidine tetrahydrochloride [DAB, the chromogen] in Tris-HCl; UltraVision Detection Systems, Thermo Scientific) was applied to the slides for 30 s. The sections were washed in distilled water, counterstained with hematoxylin, cover slipped, and examined under an Olympus CX31 microscope. All analyses were carried out in a minimum of 100 randomly selected seminiferous tubules in four animals per group.
Serum and intratesticular, testosterone, and E2 analyses
Ninety-day-old male mice from each group were anesthetized intraperitoneally (IP), with ketamine (100 mg kg−1)/xylazine (10 mg kg−1; Drag Pharma, Santiago, Chile). By cardiac puncture, blood was drawn and serum was isolated. Seminiferous tubular fluid (STF) was isolated from the whole testes according to a previous protocol.44 For each testis, 5 μl of STF was diluted in 300 μl PBS (pH 7.4). Testosterone and E2 levels in serum and STF were assessed by radioimmunoassay (RIA). All samples were processed simultaneously and ran in duplicate (n = 4 animals per group). The sensitivity and intra- and inter-assay coefficients of variation (CVs) for the testosterone assay were 4.93 pg per tube and <13.4% and <7.6%, respectively. For the E2 assay, the sensitivity was 2.25 pg per tube and the intra- and inter-assay CVs were <11.3% and <24.9%, respectively.
Messenger RNA (mRNA) expression by quantitative polymerase chain reaction (qPCR)
RNA was isolated from decapsulated testes of 90-day-old mice (n = 3 animals per group) by using TRIzol® Reagent (Life Technologies Corporation, Carlsbad, CA, USA). Concentration and quality control were determined by analyzing the optical density ratios of 260/280 and 260/230 obtained from an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). Then, reverse transcription was performed on 500 ng total RNA with Oligo dT17, 1× first-strand buffer (Invitrogen, Carlsbad, CA, USA), 0.01 mol l−1 dithiothreitol (DTT), 0.1 mmol l−1 each dNTP, 200 U superscript II (Invitrogen), and RNase-free water (20 μl final reaction volume). qPCR was performed with 10 μl 2 × SYBR Green PCR Supermix (Bio-Rad, Hercules, CA, USA), 2 μl complementary DNA (cDNA) template, and 0.0625 μmol l−1 each specific primer for aromatase (Cyp19a1; forward: 5′-GACAGGCACCTTGTGGAAAT-3′ and reverse: 5′-GAGGTTCACGCCACCTACTC-3′) in a 20 μl reaction volume. PCR profiles were obtained by the iQ5 Detection System (Bio-Rad). Data were normalized by the 2−ΔΔCt method,45 with glyceraldehyde-3-phosphate dehydrogenase (Gapdh; forward: 5′-GCTGATGCTCCCATGTTCGTGAT-3′ and reverse: 5′-GTGGTGCAAGAGGCATTGCTGAC-3′) as the endogenous reference gene.
Cholesterol and fatty acid analyses
Testis from the HFD-fed or chow-fed mice (90-day-old mice, n = 4 animals per group) were used for cholesterol and fatty acid analyses. A modification of the Bligh and Dyer method46 was used for fatty acid and cholesterol extraction. Fatty acids were cold-esterified with potassium hydroxide in methanol. Methyl esters of fatty acids and cholesterol were analyzed by gas chromatography on a Clarus 500 chromatograph (Perkin Elmer, Shelton, CT, USA), equipped for fatty acids with an autosampler, SPTM Fused Silica Capillary Column 2380 (60 m × 0.25 mm × 0.2 μm film thickness; Supelco, Bellefonte, PA, USA), and a DB-17 column (30 m × 0.25 mm × 0.15 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). For cholesterol, a flame ionization detector (FID) was used with nitrogen as a carrier gas.
Sperm preparation and quantification
Dissected cauda epididmys from the HFD-fed or chow-fed mice (90-day-old mice, n = 4 per group) were cut into small sections and then transferred in 3 ml of noncapacitating medium (NCM), containing 86.8 mmol l−1 NaCl, 4.17 mmol l−1 KCl, 3.5 mmol l−1 CaCl2·2H2O, 1.22 mmol l−1 MgSO4·7H2O, 1.22 mmol l−1 KH2 PO4, 50.28 mmol l−1 HEPES, 5.6 mmol l−1 glucose, 521 mmol l−1 pyruvic acid, 45 mmol l−1 DL-lactic acid, 0.06 mg ml−1 penicillin, 0.05 mg ml−1 streptomycin, and 0.01 g l−1 phenol red. This medium was prepared in the absence of bovine serum albumin (BSA) and Na2 HCO3.47 The spermatozoa were allowed to freely swim into the media for 5 min at 37°C with 5% (v/v) CO2. The sperm concentration was determined in a Neubauer chamber. Samples with >80% motility were used for capacitation experiments.
Sperm capacitation and AR
To examine capacitation, the spermatozoa were suspended in 200 μl standard capacitating medium (CM), containing 72 mmol l−1 NaCl, 4.17 mmol l−1 KCl, 3.5 mmol l−1 CaCl2·2H2O, 1.22 mmol l−1 MgSO4·7H2O, 1.22 mmol l−1 KH2 PO4, 23.53 mmol l−1 HEPES, 25.7 mmol l−1 Na2 HCO3, 5.6 mmol l−1 glucose, 521 mmol l−1 pyruvic acid, 45 mmol l−1 DL-lactic acid, 0.06 mg ml−1 penicillin, 0.05 mg ml−1 streptomycin, 12 mg ml−1 BSA, and 0.01 g l−1 phenol red, or NCM and incubated for 2 h at 37°C with 5% CO2. For time course capacitation experiments, the spermatozoa were incubated in CM or NCM in the previously described conditions for 0 min, 15 min, 30 min, 60 min, or 120 min.
The AR was induced by incubating spermatozoa for 15 min in CM or NCM with 40 μmol l−1 progesterone (P4). In addition, to determine the maximum percentage of spermatozoa capable of undergoing the AR, an aliquot of cells was incubated with 10 μmol l−1 of A23187, a calcium ionophore (Sigma-Aldrich, St. Louis, MO, USA), for 1 h at 37°C in 5% CO2. Then, sperm suspensions were collected and fixed with 4% (w/v) paraformaldehyde in PBS (pH 7.4) for 30 min at 4°C. The AR was evaluated by the Coomassie blue dye technique.48,49 In brief, fixed spermatozoa were washed three times with 100 mmol l−1 ammonium acetate by centrifugation at 1210 g, layered onto microscope glass slides, and dried at 30°C. The slides were washed in distilled water and methanol for 5 min at room temperature, submerged in Coomassie brilliant blue G-250 (Winkler, Santiago, Chile) solution (0.22% [w/v] Coomassie brilliant blue in 50% [w/v] methanol and 10% [w/v] acetic acid) for 2 min, washed with distilled water, and mounted on Entellan® (Merck, Darmstadt, Germany). All samples were observed under an Olympus CX31 phase-contrast light microscope with a ×40 objective lens, and at least 200 cells were assessed for acrosomal negative or positive staining in spontaneous (without P4) or P4- or A23187-induced AR. In addition, differences between the percentage of progesterone-induced AR (P4-AR) and spontaneous AR (SP-AR) and the rate of AR after progesterone challenger (ARPC = [P4-AR] − [SP-AR]) from four mice were measured.
Sperm ultrastructural analysis
The sperm were fixed in a mixture of 4% (w/v) paraformaldehyde and 0.1% (w/v) glutaraldehyde in PBS (pH 7.4), dehydrated in ethanol, and embedded in LR White resin (London Resin Company, Stansted, UK). Semi-thin sections were obtained by ultramicrotomy, and ultrastructural analysis of sperm head with normal morphology was done under a Philips Tecnai 12 electron microscope (BioTWIN, Eindhoven, The Netherlands).
Sperm protein extraction and western blotting
Sperm protein was extracted by homogenizing sperm suspensions in a buffer containing 1 mol l−1 NaCl, 1 mmol l−1 ethylenediaminetetraacetic acid (EDTA), 10 mg ml−1 phenylmethylsulfonyl fluoride (PMSF), 1% (v/v) Triton X-100, 20 mmol l−1 Tris-HCl (pH 7.4), and a protease inhibitor cocktail (Sigma-Aldrich) – including 2 mmol l−1 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), 0.3 mmol l−1 aprotinin, 130 mmol l−1 bestatin hydrochloride, 14 mmol l−1 E-64, 1 mmol l−1 EDTA, and 1 mmol l−1 leupeptin hemisulfate. The homogenate was centrifuged for 10 min at 10 000 g at 4°C.49 Proteins were resolved on a 7% (w/v) polyacrylamide gel using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and denaturing conditions and then transferred to nitrocellulose at 400 mA for 2 h. The nitrocellulose membrane was incubated with 5% (w/v) nonfat milk and 0.1% (w/v) Tween 20 in PBS (pH 7.4) for 1 h to block nonspecific binding. Then, the membrane was incubated overnight at 4°C with a mouse monoclonal antibody against phosphotyrosine (PY20; catalog no. ab10321, Abcam, Cambridge, UK) diluted at 1:15 000 or mouse anti-β-tubulin (catalog no. 322600, Thermo Fisher) diluted at 1:5000. All antibodies were diluted in blocking solution. The membranes were subsequently washed three times and incubated with a goat anti-mouse IgG conjugated with horseradish peroxidase (KPL) diluted at 1:5000 with blocking solution for 2 h at room temperature. Peroxidase activity was detected with ECL substrate (Pierce Biotechnology, Rockford, IL, USA).
Evaluation of fertility
Ninety-day-old male mice from each group were randomly selected to evaluate their potentially fertility. Each male mouse (n = 10 males per group) was placed in a cage with two females (not exposed to any treatment, 20 females in total); all mice were then fed with the control diet and housed for 8 days (to ensure at least two complete estrous cycles). Copulation was assessed by detection of the vaginal plug, and effective pregnancy was determined by body weight increase. Between 18- and 20-day postdetection of the vaginal plug, the females were killed to quantify the number of implantations and resorptions in each uterine horn. Each ovary was dissected, placed in Bouin's solution, and embedded in paraffin. Serial 7-μm histological sections were stained with hematoxylin–eosin, and the number of luteal bodies per ovary was assessed. Specifically, luteal bodies from an ovarian section were identified and counted, and then the total number of luteal bodies counted was normalized to the total number of sections counted. Thus, all the luteal bodies of each ovary were considered. The gestational rate, fertility index, fertility potential, number of implantations, number of resorptions, preimplantation loss, and fetal mortality (number of resorptions/number of implantations) were determined as described previously.50
Statistical analyses
All data were presented as mean ± standard deviation (s.d.). To determine differences between averages, an unpaired t-test, Chi-squared test, or Wilcoxon signed-rank test was performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). Correlation between variables was measured by using Pearson's correlation coefficient (Pearson's r). P < 0.05 was considered statistically significant.
RESULTS
HFD intake and testicular histology and germ cell apoptosis
Adult HFD-fed mice began to present a higher body weight at postnatal day 77 than chow-fed mice (control group; Figure 1a). The basal blood glucose level was similar in both groups, but 120 min after the glucose tolerance test, HFD-fed mice showed elevated glucose compared with chow-fed mice (Figure 1b). HFD-fed mice presented a large accumulation of fatty tissue around the gonads compared with controls (Figure 1c). However, testicular and epididymal weights had no changes in both groups (Supplementary Table 2). Histological evaluation of the testis showed a robust decrease in the diameter of seminiferous tubules and epithelial height in HFD-fed compared with chow-fed mice Figure 2a. In addition, the HFD-fed mice exhibited significantly higher percentage of seminiferous tubules with diverse types of damage, such as exfoliated germ cells toward the tubular lumen, tubules without a lumen, and tubules with vacuoles than controls (Figure 2a). Furthermore, the testes of HFD-fed mice showed a higher rate of germ cell apoptosis than chow-fed mice, evaluated by pyknotic and caspase-3-positive cells (Figure 2b). Interestingly, there were no variations in the frequency of the seminiferous tubule stages between the groups (Figure 2c). Overall, these data suggest that HFD induced an increase in adiposity, weight gain, and prediabetic condition, all of which were associated with testicular injury accompanied by germ cell death.
Figure 1.
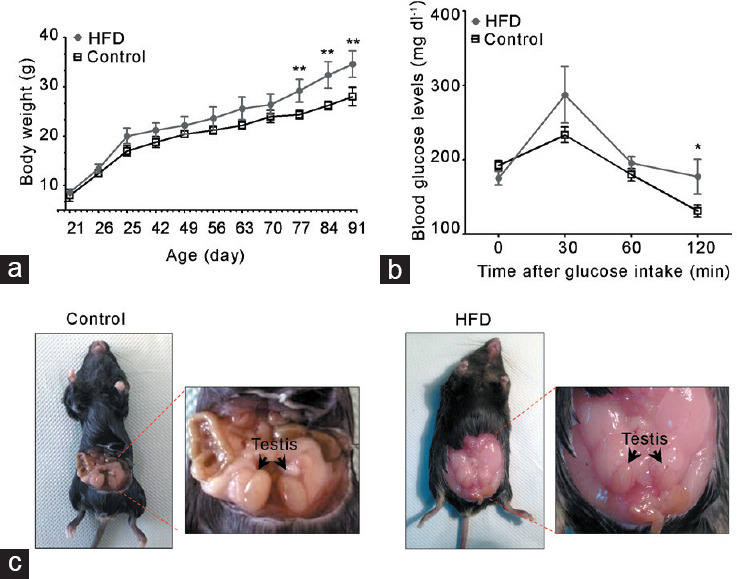
HFD feeding induced a metabolic state associated with alterations in reproductive organs. Metabolic evaluation of HFD-fed compared with chow-diet (control) mice: (a) body weight and (b) glucose tolerance test. (c) Representative images of accumulation of fatty tissue in the abdominal cavity of HFD-fed mice. All graphs represent the mean ± s.d., n = 4. Data were statistically analyzed with an unpaired t-test: *P < 0.05, **P < 0.01. s.d.: standard deviation; HFD: high-fat diet.
Supplementary Table 2.
Absolute and relative weights of the testis and epididymis
| Group |
Absolute weight (mg) |
Relative weight (organ weight/BW) |
||
|---|---|---|---|---|
| Testis | Epididymis | Testis | Epididymis | |
| Control | 93.67±4.75 | 35.27±6.51 | 0.34±0.03 | 0.13±0.02 |
| HDF | 93.07±6.75 | 29.38±0.38 | 0.25±0.06* | 0.09±0.01* |
Data are expressed as mean±s.d., n=3 per group. *P<0.05 between the HFD-fed and chow-fed mice using an unpaired t-test. BW: body weight; s.d.: standard deviation; HFD: high-fat diet
Figure 2.
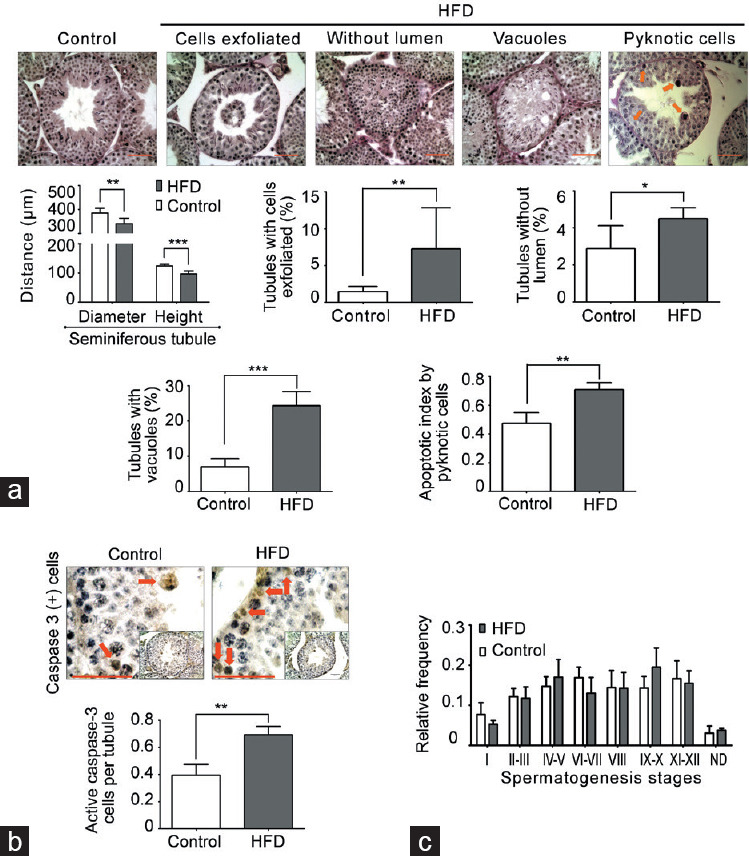
Degeneration-atrophy of seminiferous tubules and increase in apoptosis in HFD-fed mice. (a) Morphometric analysis of seminiferous tubules (diameter and epithelial height). In addition, images of testicular sections stained with PAS and hematoxylin that present different types of seminiferous tubule degeneration/atrophy and germ cell death by pyknotic cells (orange arrow) found in HFD-fed compared with chow-fed mice. The quantification of histological alterations and germ cell death in HFD-fed versus chow-fed mice is shown at the bottom. Scale bars = 100 μm. (b) Evaluation of germ cell apoptosis by caspase-3-positive cells (red arrow). Scale bars = 100 μm. (c) Frequency of seminiferous epithelial cycles. All graphs represent the mean ± s.d., n = 4. Data were statistically analyzed with an unpaired t-test: *P < 0.05, **P < 0.01, ***P < 0.001. s.d.: standard deviation; HFD: high-fat diet; PAS: periodic acid–Schiff.
Changes in intratesticular E2 in HFD-fed mice
Given that we observed an increase in seminiferous tubule degeneration without changes in testicular and epididymal weights in HFD-fed mice, we examined whether these alterations were due to changes in hormone levels. Serum and intratesticular testosterone levels were similar between HFD-fed and chow-fed mice (Figure 3a). However, the intratesticular E2 level and E2-to-testosterone ratio were lower in HFD-fed mice (Figure 3b and 3c). This phenomenon did not seem to be due to a deficiency in the production of E2 because the mRNA level of Cyp19a1 that encodes aromatase, the enzyme that produces E2 from testosterone, was not different between the two groups (Figure 3d).
Figure 3.
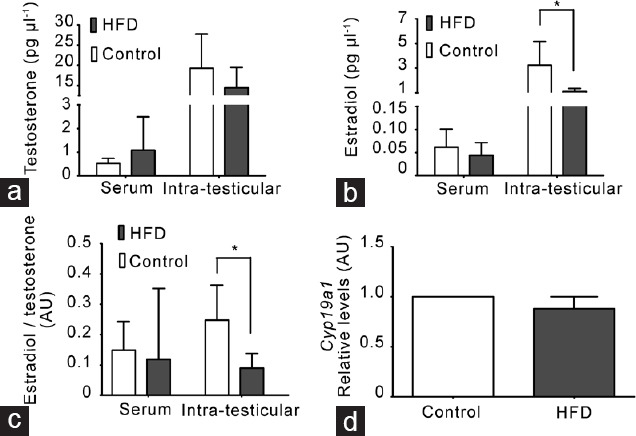
A decrease in intratesticular estradiol (E2) levels was not related to changes in E2 synthesis in mice fed with a HFD. (a) Testosterone levels in serum and seminiferous tubular fluid (intratesticular). (b) E2 levels in serum and seminiferous tubular fluid (intratesticular). (c) E2-to-testosterone ratio in serum and seminiferous tubular fluid (intratesticular). All measurements were made by radioimmunoassay. All graphs represent the mean ± s.d., n = 4. Data were statistically analyzed with an unpaired t-test: *P < 0.05. (d) Cyp19a1 (aromatase) gene expression by quantitative polymerase chain reaction in adult mouse testes at the experimental end point. All graphs represent the mean ± s.d., n = 3. Data were analyzed by Wilcoxon signed-rank test. AU: arbitrary units; s.d.: standard deviation; HFD: high-fat diet.
Cholesterol and fatty acid regulation in HFD-fed mouse testes
It is plausible that the intake of a diet rich in cholesterol and saturated and unsaturated fatty acids modifies the lipid content in testicular cells, but this has not been fully elucidated. First, we characterized the total cholesterol and fatty acid profiles in the testis of HFD-fed and chow-fed mice. The total cholesterol content was similar in both groups (Table 1). For total fatty acids, only behenic acid (C22:0) and DHA (C22:6n-3) levels were lower in HFD-fed than chow-fed mice (Table 1). Furthermore, there were no differences in the percentage of saturated fatty acids, mono-unsaturated fatty acids (MUFA), or PUFA between HFD-fed and chow-fed mouse testes (Table 1). Thus, fine-tuned changes, especially a decrease in DHA in testis by HFD consumption, might induce significant alterations in sperm function.
Table 1.
Cholesterol and fatty acid composition in testis from high-fat diet-fed and chow-fed mice (control)
| Parameter | Control (n=4) | HFD (n=4) | P |
|---|---|---|---|
| C4:0 (%) | 0.75±0.51 (0.30–1.40) | 4.95±8.13 (0.30–17.10) | 0.3423 |
| C6:0 (%) | 1.00±0.61 (0.40–1.80) | 0.80±0.48 (0.50–1.50) | 0.6221 |
| C8:0 (%) | 0.73±0.86 (0.202.00) | 0.30±0.16 (0.10–0.50) | 0.3699 |
| C16:0 (%) | 22.75±1.86 (20.50–24.80) | 18.10±3.59 (13.90–21.60) | 0.0609 |
| C18:0 (%) | 6.18±1.07 (4.90–7.20) | 5.98±1.13 (4.50–7.00) | 0.8056 |
| C18:1n9t (%) | 8.08±2.73 (6.00–12.10) | 7.95±2.28 (5.10–10.40) | 0.9563 |
| C18:1n9c (%) | 1.50±0.14 (1.30–1.60) | 1.23±0.21 (1.99–1.40) | 0.0701 |
| C18:2n6c (%) | 2.80±1.50 (1.70–5.00) | 2.57±1.42 (1.20–4.50) | 0.8351 |
| C20:3n6 (%) | 9.28±1.17 (8.10–10.80) | 8.45±2.03 (6.50–10.40) | 0.5080 |
| C22:0 (%) | 1.20±0.18 (1.00–1.40) | 0.83±0.15 (0.70–1.00) | 0.0192* |
| C24:1n9 (%) | 9.96±0.74 (9.10–10.90) | 9.13±2.20 (7.20–11.50) | 0.4919 |
| C22:6n3 (%) | 6.38±0.63 (5.60–7.10) | 3.93±0.96 (3.10–4.90) | 0.0053* |
| SFA (%) | 32.60±4.14 (28.20–37.40) | 30.95±5.01 (25.80–37.80) | 0.6295 |
| MUFA (%) | 19.55±3.01 (16.40–23.60) | 18.28±3.47 (13.20–20.80) | 0.6001 |
| PUFA (%) | 18.45±2.62 (15.30–21.20) | 14.93±3.08 (10.80–17.70) | 0.1323 |
| NI (%) | 29.40±9.09 (20.70–40.10) | 35.85±4.48 (31.80–41.00) | 0.2506 |
| Cholesterol (mg per sample) | 1.05±0.21 (0.76–1.23) | 1.31±0.12 (1.18–1.44) | 0.0746 |
| Sample (g) | 0.05 | 0.05 |
Data are expressed as mean±s.d., with the minimum and maximum values in parentheses (n=4). *Significant differences at P<0.05 level compared to control group; unpaired t-test. SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; NI: not identified; s.d.: standard deviation; HFD: high-fat diet
Changes in AR in spermatozoa from HFD-fed mice
Neither the epididymal sperm count nor the sperm head ultrastructure was different between HFD-fed and chow-fed mice (Figure 4a and 4b). Given that capacitation involves a series of biochemical changes that require an efflux of cholesterol, and previous studies have shown that the sperm plasma membrane composition is modified in obese men and mice, we evaluated whether these physiological parameters would change under this condition. One of the key steps during in vitro sperm capacitation is the change in tyrosine phosphorylation before the AR. Spermatozoa from both chow-fed and HFD-fed mice showed a sustained and similar increase in the tyrosine phosphorylation pattern after 2 h of incubation (Figure 4c). We next determined the percentage of SP-AR and P4-AR at different incubation time. The percentage of SP-AR for chow-fed and HFD-fed mice was similar between the groups throughout the incubation time, with a constant and smooth increase in both cases (Figure 4d). On the other hand, the percentage of P4-AR was higher in spermatozoa from HFD-fed than chow-fed mice at all times examined (Figure 4d). Thus, the AR induced in HFD-fed mouse spermatozoa seems more sensitive to external stimuli than that of spermatozoa from chow-fed mice. This phenomenon would have an impact on sperm-fertilizing capacity.
Figure 4.
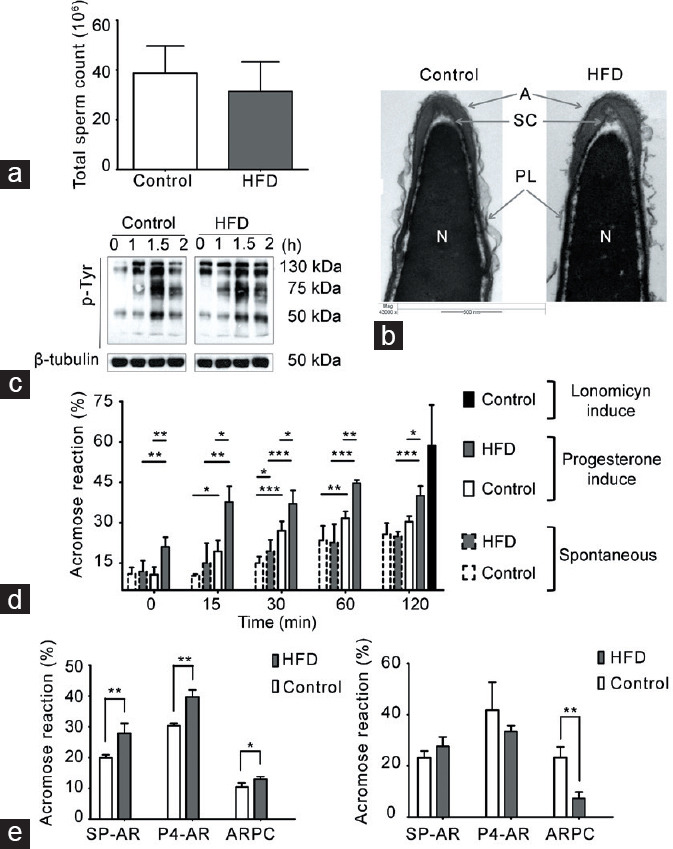
Capacitation and the AR in spermatozoa from HFD-fed mice. Comparison between HFD-fed and chow-diet (control) mice. (a) Total sperm count in cauda epididymis, graph represents the mean ± s.d., n = 4. No significant differences were observed. (b) Representative images of acrosomal ultrastructure in HFD-fed and chow-fed mice. The images depict the acrosome (A), sub-acrosomal compartment (SC), nucleus (N), and plasma membrane (PL). (c) Representative images of the time-dependent pattern of tyrosine phosphorylation; β-tubulin was used as a loading control, n = 3. (d) Quantification of AR; spermatozoa from each group were incubated in CM. The time-dependent percentage of spontaneous AR (SP-AR) and AR induced by 40 μmol l−1 of progesterone (P4-AR) was evaluated with the Coomassie blue dye technique. As a control, the maximum AR response was evaluated in the presence of 10 μmol l−1 A23187, a calcium ionophore. All graphs represent the mean ± s.d., n = 4. SP-AR and P4-AR were analyzed with an unpaired t-test: *P < 0.05, **P < 0.01, ***P < 0.001. (e) SP-AR and P4-AR and the rate of ARPC were measured with the Coomassie blue dye technique, after incubating the spermatozoa in CM with 10 mg ml−1 or 30 mg ml−1 bovine serum albumin as a cholesterol scavenger. All graphs represent the mean ± s.d., n = 4. Data were statistically analyzed with an unpaired t-test: *P < 0.05, **P < 0.01. s.d.: standard deviation; AR: acrosomal reaction; HFD: high-fat diet; CM: capacitating medium; ARPC: AR after progesterone challenger.
Previous studies have indicated that the AR depends on the cholesterol content of the spermatozoa. Hence, we evaluated whether variations in the concentration of a cholesterol scavenger (e.g., albumin) in the culture medium could modulate both SP-AR and P4-AR. The spermatozoa from HFD-fed mice incubated with 10 mg ml−1 BSA presented a significant increase in the percentage of SP-AR and P4-AR compared with chow-fed mice (Figure 4e). However, when determining the difference between the percentage of P4-AR and SP-AR, which is considered the population of spermatozoa capable of responding to P4 stimulation (ARPC), there was no difference between HFD-fed and chow-fed animals. By contrast, when incubated with a higher BSA concentration (30 mg ml−1), SP-AR and P4-AR were similar between sperm from HFD-fed and chow-fed mice (Figure 4e). However, the ARPC was significantly higher in chow-fed than that in HFD-fed mice. Overall, these data indicate that spermatozoa from obese mice have a “labile” plasma membrane that modifies its ability to undergo the AR in vitro.
We evaluated whether changes in testicular DHA were correlated with steroid hormone levels or with the AR. Testicular DHA levels were positively correlated with intratesticular E2 but not testosterone (Supplementary Figure 1a (326.3KB, tif) and 1b (326.3KB, tif) ). In addition, intratesticular DHA levels were negatively correlated with SP-AR (Supplementary Figure 1c (326.3KB, tif) ) but not with P4-AR or ARPC (Supplementary Figure 1d (326.3KB, tif) -1f (326.3KB, tif) ).
HFD intake in mice and fertility
Finally, we examined whether fertility was similar in HFD-fed and chow-fed mice. The gestation rate and fertility potential were lower in HFD-fed than chow-fed mice (Chi-squared test: P < 0.001, and Wilcoxon signed-rank test: P < 0.034). In addition, we observed that fetal offspring of the HFD-fed mice presented a higher embryo resorption (unpaired t-test; P < 0.017) and fetal mortality (unpaired t-test; P < 0.034) than the chow-fed group (Table 2). Other parameters, such as the fecundity index, implantation rates, and preimplantation loss were similar between the groups.
Table 2.
Fertility parameters in high-fat diet-fed versus chow-fed (control) mice
| Parameter | Control (n=10) | HFD (n=10) | P |
|---|---|---|---|
| Gestational rate (%)a | 100±0 | 75.00±26.73 | <0.001** |
| Fecundity index (%)a | 100±0 | 100±0 | 1.000 |
| Fertility potential (%)b | 100±0 | 92.25±8.28 | 0.034* |
| Number of implantation (gestational day 20)c | 8.30±1.16 | 8.60±3.77 | 0.813 |
| Number of resorption (gestational day 20)c | 0.10±0.32 | 0.60±0.52 | 0.017* |
| Number of luteal bodiesc | 8.30±0.95 | 9.30±3.95 | 0.605 |
| Preimplantation loss (%)c | 1.56±4.42 | 7.75±8.28 | 0.076 |
| Fetal mortality (%)c | 0.01±0.05 | 0.08±0.07 | 0.034* |
Data are expressed as mean±s.d. aChi-squared test; bWilcoxon signed-rank test; cunpaired t-test. *Significant differences at P<0.05 level compared to control group; **Significant differences at P<0.001 level compared to control group. s.d.: standard deviation
DISCUSSION
Data from human patients and animal models indicate that obesity adversely impacts male fertility. In the present study, we showed that an HFD mouse model recapitulates previous data from the literature. These findings suggest that the decreased fertility observed in this model may be partially explained by the high lability of spermatozoa to undergo the AR. This eventuality probably occurs due to downregulation of some fatty acid levels, such as DHA and behenic acid in testes.
We found that mice fed with an HFD over 2 months showed an increased germ cell apoptosis and seminiferous tubule damage. These findings are similar to previously reported changes.16,18,23,51 Interestingly, there were no changes in epididymal sperm count or the frequency of seminiferous tubule stages; these data suggest that the rate of sperm production remained constant, despite the high seminiferous tubule damage. While some work has shown that obesity promotes changes in the rate of spermatogenesis and a decrease in sperm count,18,20,21,51 others have failed to find these changes.16 We believe that the differences in our data from that in the literature may be related to the type of diet, time of treatment, or age of mice.
In the testis, testosterone exerts its effects by the activation of the androgen receptor as well as via activation of the estrogen receptor (after its aromatization to E2). The majority of studies on human and animal models have indicated an increase in serum E2 mostly related to an increase in white adipose tissue mass.52 Our data demonstrated a decrease in serum and intratesticular E2 levels along with a decrease in the E2-to-testosterone ratio, without changes in intratesticular or serum testosterone levels in HFD-fed mice. These findings could explain the similarity in sperm count and frequency of seminiferous tubule stages between the HFD-fed and chow-fed mice. Experiments of short- and long-term diet-induced obesity in rats have shown that the testicular testosterone level is reduced only after long-term diet exposure. After short-term treatment (3 months), there are only alterations in serum E2 levels and steroidogenic gene expression.53 Given that we did not observe changes in Cyp19a1 expression, the decrease in E2 may be related to deregulation in the levels of enzymes involved in its metabolism.54,55 A recent report showed elevated levels of cytochrome P450, family 1, subfamily A, member 1 (CYP1A1) and Catechol-O-methyltransferase (COMT), which produce hydroxyl-estradiol and methoxy-estradiol, respectively, in testicular biopsies of overweight patients with spermatogenic failure.56 In addition, these E2 metabolites induce DNA fragmentation in Sertoli cells.57 Thus, an increase in E2 metabolism rather than decreased E2 production could explain E2 decline without altered testosterone levels. Further studies should focus on the role of intratesticular E2 and its metabolites in obesity-induced infertility.
Previous studies have shown that spermatozoa from HFD-fed mice and rabbits show decreased capacitation compared with controls,16,58 but in the present study, we showed that the total phosphotyrosine pattern – a parameter of sperm capacitation – was similar between HFD-fed and chow-fed mice. It is possible that the differences between our findings and the previous results are due to different diet composition or the methodology to evaluate capacitation. The AR, the fusion between the outer acrosomal membrane and the plasma membrane, can occur spontaneously, probably as a consequence of sperm death, or after some stressor or condition that promotes the fusion between plasma and acrosomal membranes. On the other hand, the so-called “physiological” or “true” reaction is one that occurs only after the completion of training and against some stimulus that the spermatozoa would find in the genital tract – progesterone is most used in vitro.59,60,61 In this study, there was a negative correlation between testicular DHA levels and SP-AR but not P4-AR. Although it is true that we did not quantify the levels of DHA in spermatozoa, a study in pig sperm showed results similar to ours.62 Interestingly, the male D6D-null mouse that is unable to synthesize PUFAs, including AA and DHA, and a mouse null for acyl-CoA synthetase isoform 6 (ACSL6), which activates DHA by ligating a CoA, are infertile; DHA diet supplementation restores spermatogenesis and fertility in that model.37 DHA is required during spermiogenesis for acrosomal formation related to transport of syntaxin and acrosine from the endoplasmic reticulum (ER) to the Golgi apparatus and Golgi-derived vesicle fusion.63 Furthermore, this negative correlation could be explained by the fact that DHA affects the fluid properties of biological membranes owing to the extent of its polyunsaturation, and that it is highly enriched in the head of human spermatozoa.64 This hypothesis could explain why the total SP-AR and P4-AR were higher in the spermatozoa of HDF-fed than chow-fed mice, and not with a medium with a high concentration of albumin. BSA is a cholesterol scavenger and promotes fluidity and fusion of the membranes; these phenomena occur in the spermatozoa of chow-fed but not HFD-fed mice. The “true” AR, the one that is induced by progesterone, which in this work we call ARPC, was higher in spermatozoa from HFD-fed than chow-fed mice, but the opposite occurred when the albumin concentration was increased. This could be explained as a reflection of the changes in the lipid composition between the spermatozoa plasma membranes from the different groups. Interestingly, it has been reported that spermatozoa from obese patients show elevated cholesterol levels and an increase in SP-AR but a reduction in P4-AR.33 In humans, studies have indicated a correlation between DHA levels with various sperm parameters such as motility, viability, and concentration, but there is no study that has correlated it with either spontaneous or induced AR.34,64,65,66
In this study, we showed that HFD-fed mice had a reduced gestational rate, reduced potential fertility, and increased embryo mortality. Previous results have shown that spermatozoa from obese mice produce low-quality embryos with less potential to develop.67,68,69 Furthermore, paternal obesity in humans leads to a low fertilization rate, reduces the live birth rate by assisted reproductive technology, and increases the risk of nonviable pregnancy.70,71,72
CONCLUSION
This study demonstrated that in an obese mouse model, induced by high-fat intake, there was a decrease in intratesticular E2 related with testicular damage, especially a decrease in DHA, associated with an increase in spontaneous AR, along with affected fertility.
AUTHOR CONTRIBUTIONS
JB and RDM designed the research, performed the research, and wrote the manuscript JB and NS contributed new analytic tools; JB, LMG, JLT-F, MVA-A, RO, and RDM analyzed data. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Testicular DHA levels correlated with testicular estradiol (E2) levels and the AR. (a and b) Scatterplot for correlation between testicular DHA levels and intratesticular steroid hormone levels (testosterone and E2). (c–f) Scatterplots for correlation between testicular DHA levels and the AR (spontaneous, progesterone induced, or ARPC). A correlation was considered statistically significant when P ≤ 0.05. r: Pearson's correlation coefficient; AU: arbitrary units; DHA: docosahexaenoic acid; AR: acrosomal reaction; ARPC: AR after progesterone challenger.
ACKNOWLEDGMENTS
This work was supported by grants from FONDECYT (1150352) and CONICYT (21120505), Chile.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Esteves SC, Hamada A, Kondray V. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet. 2012;286:217–29. doi: 10.1007/s00404-012-2274-x. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedro J, Brandão T, Schmidt L, Costa ME, Martins MV. What do people know about fertility.A systematic review on fertility awareness and its associated factors? Ups J Med Sci. 2018;123:71–81. doi: 10.1080/03009734.2018.1480186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis LO, Dias FG. Male fertility, obesity, and bariatric surgery. Reprod Sci. 2012;8:778–85. doi: 10.1177/1933719112440053. [DOI] [PubMed] [Google Scholar]
- 5.Abiad F, Awwad J, Abbas HA, Zebian D, Ghazeeri G. Management of weight loss in obesity-associated male infertility: a spotlight on bariatric surgery. Human Fertil (Camb) 2017;20:227–35. doi: 10.1080/14647273.2017.1317369. [DOI] [PubMed] [Google Scholar]
- 6.Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. Hum Reprod Update. 2011;17:667–83. doi: 10.1093/humupd/dmr017. [DOI] [PubMed] [Google Scholar]
- 7.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2009;16:293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 9.Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril. 2014;102:1268–73. doi: 10.1016/j.fertnstert.2014.07.1212. [DOI] [PubMed] [Google Scholar]
- 10.Dupont C, Faure C, Sermondade N, Boubaya M, Eustache F, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013;15:622–5. doi: 10.1038/aja.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers TJ, Anderson RA. The impact of obesity on male fertility. Hormones. 2015;14:563–8. doi: 10.14310/horm.2002.1621. [DOI] [PubMed] [Google Scholar]
- 12.Colaci DS, Afeiche M, Gaskins AJ, Wright DL, Toth TL, et al. Men's body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–9. doi: 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen RH, Wilcox AJ, Skjærven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–93. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 14.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–2. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 15.Taha EA, Sayed SK, Gaber HD, Abdel Hafez HK, Ghandour N, et al. Does being overweight affect seminal variables in fertile men? Reprod Biomed Online. 2016;33:703–8. doi: 10.1016/j.rbmo.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Fan Y, Liu Y, Xue K, Gu G, Fan W, et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 2015;10:e0120775. doi: 10.1371/journal.pone.0120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao J, Pennington KA, Talton OO, Schulz LC, Sutovsky M, et al. In utero and postnatal exposure to high fat, high sucrose diet suppressed testis apoptosis and reduced sperm count. Sci Rep. 2018;8:7622. doi: 10.1038/s41598-018-25950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mia XL, Gao GM, Jiang L, Xu R, Wan DP. Asiatic acid attenuates high-fat diet-induced impaired spermatogenesis. Exp Ther Med. 2018;15:2397–403. doi: 10.3892/etm.2017.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer NO, Bakos HW, Owens JA, Setchell BP, Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metab. 2012;302:E768–80. doi: 10.1152/ajpendo.00401.2011. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Cai Y, Shao Y, Zhang X, Li N, et al. Fish oil ameliorates high-fat diet induced male mouse reproductive dysfunction via modifying the rhythmic expression of testosterone synthesis related genes. Int J Mol Sci. 2018;19:pii: E1325. doi: 10.3390/ijms19051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh S, Mukherjee S. Testicular germ cell apoptosis and sperm defects in mice upon long-term high fat diet feeding. J Cell Physiol. 2018;233:6896–909. doi: 10.1002/jcp.26581. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Liu X, Wang L, Sheng N. Brown adipose tissue transplantation ameliorates male fertility impairment caused by diet-induced obesity. Obes Res Clin Pract. 2017;11:198–205. doi: 10.1016/j.orcp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Mu Y, Yan WJ, Yin TL, Zhang Y, Li J, et al. Diet-induced obesity impairs spermatogenesis: a potential role for autophagy. Sci Rep. 2017;7:43475. doi: 10.1038/srep43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto AP, et al. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32. doi: 10.1186/1477-7827-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Incardona JP, Eaton S. Cholesterol in signal transduction. Curr Opin Cell Biol. 2000;12:193–203. doi: 10.1016/s0955-0674(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 26.Jefcoate CR, Lee J. Cholesterol signaling in single cells: lessons from STAR and sm-FISH. J Mol Endocrinol. 2018;60:R213–35. doi: 10.1530/JME-17-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gervasi MG, Visconti PE. Chang's meaning of capacitation: a molecular perspective. Mol Reprod Dev. 2016;83:860–74. doi: 10.1002/mrd.22663. [DOI] [PubMed] [Google Scholar]
- 28.Belmonte SA, Mayorga LS, Tomes CN. The molecules of sperm exocytosis. Adv Anat Embryol Cell Biol. 2016;220:71–92. doi: 10.1007/978-3-319-30567-7_4. [DOI] [PubMed] [Google Scholar]
- 29.Brewis IA, Moore HD, Fraser LR, Holt WV, Baldi E, et al. Molecular mechanisms during sperm capacitation. Hum Fertil (Camb) 2005;8:253–61. doi: 10.1080/14647270500420178. [DOI] [PubMed] [Google Scholar]
- 30.Visconti PE, Ning X, Forne MW, Alvarez JG, Stein P, et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;443:429–43. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- 31.Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, et al. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–26. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- 32.Leahy T, Gadella BM. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J Androl. 2015;17:561–7. doi: 10.4103/1008-682X.153309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samavat J, Natali I, Degl'Innocenti S, Filimberti E, Cantini G, et al. Acrosome reaction is impaired in spermatozoa of obese men: a preliminary study. Fertil Steril. 2014;102:1274–81.e2. doi: 10.1016/j.fertnstert.2014.07.1248. [DOI] [PubMed] [Google Scholar]
- 34.Andersen JM, Rønning PO, Herning H, Bekken SD, Haugen TB, et al. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology. 2016;4:857–65. doi: 10.1111/andr.12227. [DOI] [PubMed] [Google Scholar]
- 35.Roqueta-Rivera M, Stroud CK, Haschek WM, Akare SJ, Segre M, et al. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J Lipid Res. 2010;51:360–7. doi: 10.1194/jlr.M001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saether T, Tran TN, Rootwelt H, Christophersen BO, Haugen TB. Expression and regulation of delta5-desaturase, delta6-desaturase, stearoyl-coenzyme A (CoA) desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. Biol Reprod. 2003;69:117–24. doi: 10.1095/biolreprod.102.014035. [DOI] [PubMed] [Google Scholar]
- 37.Hale BJ, Fernandez RF, Kim SQ, Diaz VD, Jackson SN, et al. Acyl-CoA synthetase 6 enriches seminiferous tubules with the ω-3 fatty acid docosahexaenoic acid and is required for male fertility in the mouse. J Biol Chem. 2019;294:14394–405. doi: 10.1074/jbc.RA119.009972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen JM, Rønning PO, Herning H, Bekken SD, Haugen TB, et al. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology. 2016;4:857–65. doi: 10.1111/andr.12227. [DOI] [PubMed] [Google Scholar]
- 39.Paillamanque J, Madrid C, Carmona EM, Osses N, Moreno RD, et al. Effects of fatty acids on intracellular [Ca2+], mitochondrial uncoupling and apoptosis in rat pachytene spermatocytes and round spermatids. PLoS One. 2016;11:e0158518. doi: 10.1371/journal.pone.0158518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–32. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 41.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, et al. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol. 2012;40:40S–121S. doi: 10.1177/0192623312454337. [DOI] [PubMed] [Google Scholar]
- 42.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Int J Androl. 1993;16:83. [Google Scholar]
- 43.Urriola-Muñoz P, Lagos-Cabre R, Moreno RD. A mechanism of male germ cell apoptosis induced by bisphenol-A and nonylphenol involving ADAM17 and p38 MAPK activation. PLoS One. 2014;9:e113793. doi: 10.1371/journal.pone.0113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intra-testicular fluid. J Androl. 2001;22:640–5. [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Biochem Cell Biol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 47.Torres-Fuentes JL, Rios M, Moreno RD. Involvement of a P2X7 receptor in the acrosome reaction induced by ATP in rat spermatozoa. J Cell Physiol. 2015;230:3068–75. doi: 10.1002/jcp.25044. [DOI] [PubMed] [Google Scholar]
- 48.Bendahmane M, Zeng HT, Tulsiani DR. Assessment of acrosomal status in rat spermatozoa: studies on carbohydrate and non-carbohydrate agonists. Arch Biochem Biophys. 2002;404:38–47. doi: 10.1016/s0003-9861(02)00278-3. [DOI] [PubMed] [Google Scholar]
- 49.Cisternas P, Moreno RD. Comparative analysis of apoptotic pathways in rat, mouse, and hamster spermatozoa. Mol Reprod Dev. 2006;73:1318–25. doi: 10.1002/mrd.20561. [DOI] [PubMed] [Google Scholar]
- 50.Garcia PV, Barbieri MF, Perobelli JE, Consonni SR, Mesquita SD, et al. Morphometric-stereological and functional epididymal alterations and a decrease in fertility in rats treated with finasteride and after a 30-day post-treatment recovery period. Fertil Steril. 2012;97:1444–51. doi: 10.1016/j.fertnstert.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Ibáñez CA, Erthal RP, Ogo FM, Peres MN, Vieira HR, et al. A high fat diet during adolescence in male rats negatively programs reproductive and metabolic function which is partially ameliorated by exercise. Front Physiol. 2017;8:807. doi: 10.3389/fphys.2017.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katib A. Mechanisms linking obesity with male infertility. Cent Eur J Urol. 2015;68:79–85. doi: 10.5173/ceju.2015.01.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner IV, Klöting N, Atanassova N, Savchuk I, Spröte C, et al. Prepubertal onset of obesity negatively impacts on testicular steroidogenesis in rats. Mol Cell Endocrinol. 2016;437:154–62. doi: 10.1016/j.mce.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 54.Mauras N, Santen RJ, Colón-Otero G, Hossain J, Wang Q, et al. Estrogens and their genotoxic metabolites are increased in obese prepubertal girls. J Clin Endocrinol Metab. 2015;100:2322–8. doi: 10.1210/jc.2015-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider J, Bradlow HL, Strain G, Levin J, Anderson K, et al. Effects of obesity on oestradiol metabolism: decreased formation of nonuterotropic metabolites. J Clin Endocrinol Metab. 1983;56:973–8. doi: 10.1210/jcem-56-5-973. [DOI] [PubMed] [Google Scholar]
- 56.Parada-Bustamante A, Molina C, Valencia C, Flórez M, Lardone MC, et al. Disturbed testicular expression of the estrogen-metabolizing enzymes CYP1A1 and COMT in infertile men with primary spermatogenic failure: possible negative implications on Sertoli cells. Andrology. 2017;3:486–94. doi: 10.1111/andr.12346. [DOI] [PubMed] [Google Scholar]
- 57.Valencia C, Molina C, Florez M, Buñay J, Moreno RD, et al. 2-hydroxyooestradiol and 2-methoxyooestradiol, two endogenous oestradiol metabolites, induce DNA fragmentation in Sertoli cells. Andrologia. 2016;48:1294–306. doi: 10.1111/and.12576. [DOI] [PubMed] [Google Scholar]
- 58.Saez Lancellotti TE, Boarelli PV, Romero AA, Funes AK, Cid-Barria M, et al. Semen quality and sperm function loss by hypercholesterolemic diet was recovered by addition of olive oil to diet in rabbit. PLoS One. 2013;8:e52386. doi: 10.1371/journal.pone.0052386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calogero AE, Burrello N, Barone N, Palermo I, Grasso U, et al. Effects of progesterone on sperm function: mechanisms of action. Hum Reprod. 2000;15:28–45. doi: 10.1093/humrep/15.suppl_1.28. [DOI] [PubMed] [Google Scholar]
- 60.Simons J, Fauci L. A model for the acrosome reaction in mammalian sperm. Bull Math Biol. 2018;80:2481–501. doi: 10.1007/s11538-018-0478-3. [DOI] [PubMed] [Google Scholar]
- 61.Tomes CN. The proteins of exocytosis: lessons from the sperm model. Biochem J. 2015;465:359–70. doi: 10.1042/BJ20141169. [DOI] [PubMed] [Google Scholar]
- 62.Lee SH, Kim YJ, Kang BH, Yun YS, Park CK. The relationship between acrosome reaction and polyunsaturated fatty acid composition in boar sperm. Reprod Domest Anim. 2020;55:624–31. doi: 10.1111/rda.13661. [DOI] [PubMed] [Google Scholar]
- 63.Roqueta-Rivera M, Abbott TL, Sivaguru M, Hess RA, Nakamura MT. Deficiency in the omega-3 fatty acid pathway results in failure of acrosome biogenesis in mice. Biol Reprod. 2011;85:721–32. doi: 10.1095/biolreprod.110.089524. [DOI] [PubMed] [Google Scholar]
- 64.Valentine RC, Valentine DL. Omega-3 fatty acids in cellular membranes: a unified concept. Prog Lipid Res. 2004;43:383–402. doi: 10.1016/j.plipres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Hosseini B, Nourmohamadi M, Hajipour S, Taghizadeh M, Asemi Z, et al. The effect of omega-3 fatty acids, EPA, and/or DHA on male infertility: a systematic review and meta-analysis. J Diet Suppl. 2019;16:245–56. doi: 10.1080/19390211.2018.1431753. [DOI] [PubMed] [Google Scholar]
- 66.Lan L, Harrison CL, Misso M, Hill B, Teede HJ, et al. Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women. Hum Reprod. 2017;32:1925–40. doi: 10.1093/humrep/dex241. [DOI] [PubMed] [Google Scholar]
- 67.Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–10. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 68.Binder NK, Mitchell M, Gardner DK. Parental diet-induced obesity leads to retarded early mouse embryo development and altered carbohydrate utilisation by the blastocyst. Reprod Fertil Dev. 2012;24:804–12. doi: 10.1071/RD11256. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell M, Bakos HW, Lane M. Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril. 2011;95:1349–53. doi: 10.1016/j.fertnstert.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 70.Campbell JM, Lane M, Owens JA, Bakos HW. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod Biomed Online. 2015;31:593–604. doi: 10.1016/j.rbmo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 71.Yang Q, Zhao F, Hu L, Bai R, Zhang N, et al. Effect of paternal overweight or obesity on IVF treatment outcomes and the possible mechanisms involved. Sci Rep. 2016;6:29787. doi: 10.1038/srep29787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raad G, Hazzouri M, Bottini S, Trabucchi M, Azoury J, et al. Paternal obesity: how bad is it for sperm quality and progeny health? Basic and Clin Androl. 2017;27:20. doi: 10.1186/s12610-017-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Testicular DHA levels correlated with testicular estradiol (E2) levels and the AR. (a and b) Scatterplot for correlation between testicular DHA levels and intratesticular steroid hormone levels (testosterone and E2). (c–f) Scatterplots for correlation between testicular DHA levels and the AR (spontaneous, progesterone induced, or ARPC). A correlation was considered statistically significant when P ≤ 0.05. r: Pearson's correlation coefficient; AU: arbitrary units; DHA: docosahexaenoic acid; AR: acrosomal reaction; ARPC: AR after progesterone challenger.


