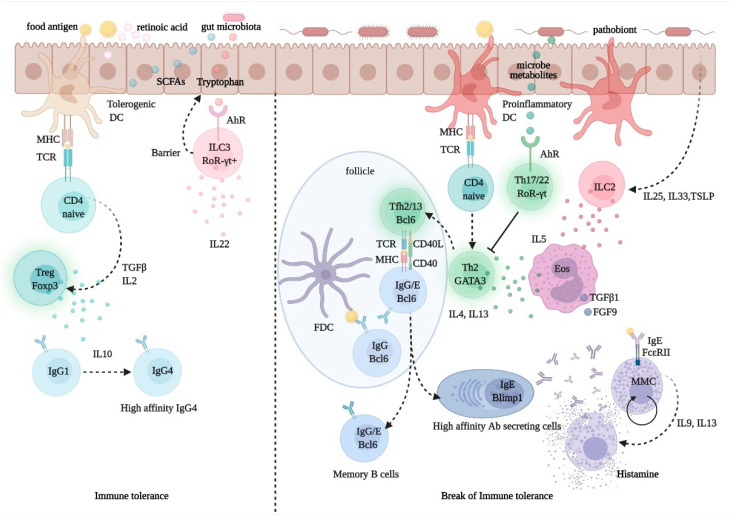
Figure 3.
Regulation of immune tolerance in the gut mucosa. Upon processing of dietary fibers, bacterial metabolites, such as short chain fatty acids (SCFA) and retinoic acid (RA), direct the development and function of FoxP3+ Treg cells via the interaction with gut epithelial cells and tolerogenic dendritic cells (DCs) with naïve CD4+ T cells. The activation and expansion of Treg cells promote the production of the immune regulatory cytokine, IL-10, which foster IgG1 to IgG4 B-cell class switching. Allergen-specific IgG4 B cells produce high-affinity antibodies for food allergens, preventing allergen interactions with mast cell-bound IgE. Microbiota-delivered factors, such as tryptophan-indole catabolites, may directly activate ROR-γt+ type-3 innate lymphoid cells (ILC3), via the aryl-hydrocarbon receptor (AhR), and induce the production of IL-22, a cytokine promoting gut epithelial regeneration and barrier integrity. Conversely, upon exposure to pathobionts, DCs and epithelial cells receive danger signals and release cytokines, such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP); these promote the activation and expansion of ILC2s, which express Th2 cytokines, such as IL4, IL-5, and IL13. While IL-5 promotes eosinophil activation and differentiation and the production of profibrotic factors, such as transforming growth factor (TGF)-β1 and fibroblast growth factor (FGF)-9, IL-13 produced by Th2 cells and T follicular helper (Tfh) 13 cells, is critical for the expression of high-affinity antigen-specific IgE. IgE antibodies interact with FcεRI on mast cells and upon exposure to allergen triggers degranulation and release of histamine, which causes allergy and inflammation.