Scheme 1. Syntheses of 1-(hydroxymethyl)-3-aryl Isoquinoline Substratesa.
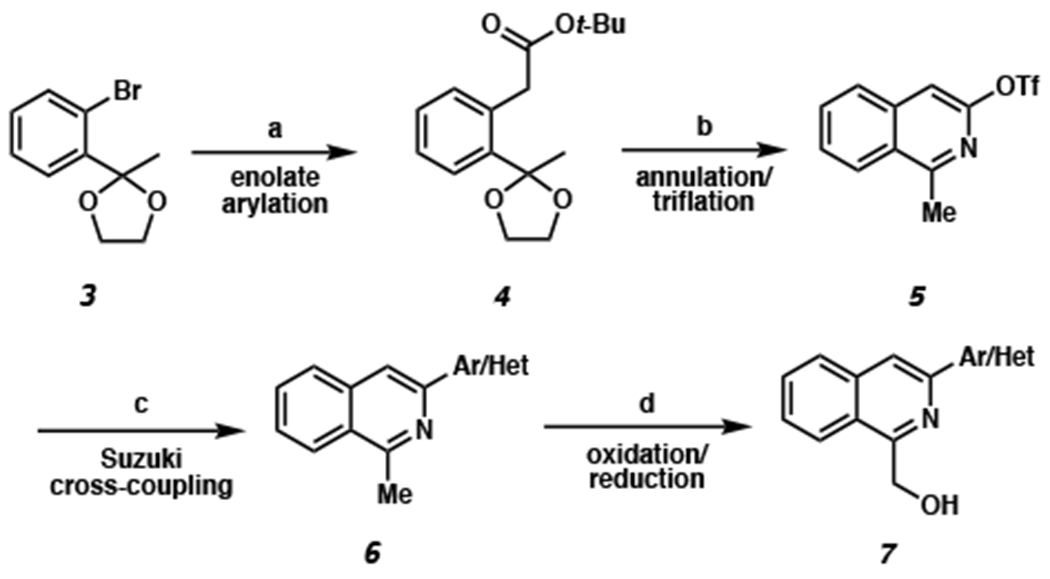
aConditions: (a) t-butyl acetate (2 equiv), LiHMDS (2.5 equiv), Pd2(dba)3 (5 mol %), P(t-Bu3)•HBF4 (10 mol %), toluene, 23 °C, 18 h, 92% yield. (b) 33% TFA in CH2Cl2, 23 °C, 2 h; then aq. NH4OH, MeCN, 70 °C, 12 h; then Tf2O (2 equiv), pyridine, CH2Cl2, 0 °C, 1 h, 38% yield over 3 steps. (c) aryl/heteroaryl boronic acid (1.5 equiv), XPhos Pd G3 (2 mol %), 2:1 K3PO4:THF, 40 °C, 2 h, 77–95% yield. (d) SeO2 (2 equiv), 1,4-dioxane, 110 °C, 2 h; then NaBH4 (1 equiv), 4:1 CH2Cl2:MeOH, 10 min, 25–97% yield.
