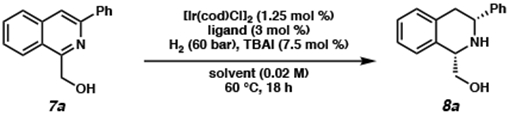
Table 1.
Optimization of the Enantioselective Hydrogenation of Isoquinolines to afford THIQsa
 | |||||
|---|---|---|---|---|---|
| entry | ligand | Solvent | % conversionb | cis:transb | % ee of cisc |
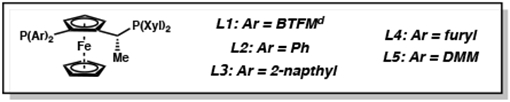
| 1 | L1 | PhMe:AcOH | >95 | >20:1 | −49d |
| 2 | L2 | PhMe:AcOH | >95 | 3.2:1 | 84 |
| 3 | L3 | PhMe:AcOH | 66 | 4.5:1 | 63 |
| 4 | L4 | PhMe:AcOH | 92 | 4.9:1 | 3 |
| 5 | L5 | PhMe:AcOH | >95 | 2.0:1 | 89 |
| 6 | – | PhMe:AcOH | >95 | >20:1 | 0 |
| 7 | L5 | CH2Cl2:AcOH | >95 | 1.5:1 | 84 |
| 8 | L5 | dioxane:AcOH | 66 | 5.5:1 | 87 |
| 9 | L5 | THF:AcOH | >95 | 9.7:1 | 90 |
| 10 | L5 | CPME:AcOH | 38 | >20:1 | 88 |
| 11 | L5 | 2-MeTHF:AcOH | >95 | 9.5:1 | 90 |
| 12e | L5 | THF:AcOH | >95 | 15.7:1 | 92 |
 | |||||
Conditions: 0.04 mmol 7a, 1.25 mol % [Ir(cod)Cl]2, 3 mol % ligand, 7.5 mol % TBAI, 60 bar H2 in 2.0 mL 9:1 solvent:AcOH.
Determined from crude 1H NMR using 1,3,5-trimethoxybenzene as standard.
Determined by chiral SFC analysis of Cbz-protected product.
Opposite enantiomer of ligand used.
Reaction performed on a 0.2 mmol scale at 23 °C, 20 bar H2, and 0.1 M concentration of 7a.
