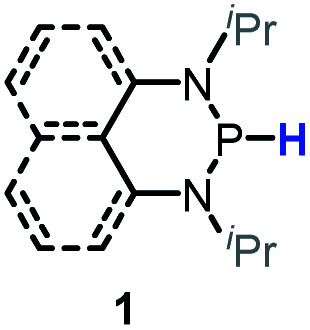
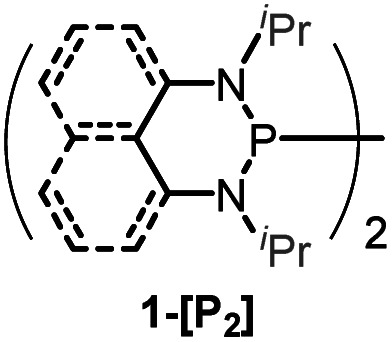
Applications of 1a and 1b in organic syntheses.
| Entry | Reactant | Condition | Product | Yield | Type |
|---|---|---|---|---|---|
| (1) |
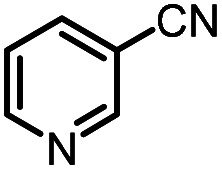
|
1a-[P]+ (15 mol%), HBpin (1.5 equiv.), CH3CN, 80 °C, 36 h |
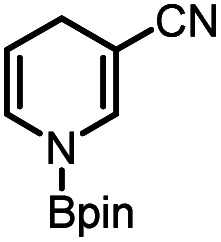
|
40%a | HT |
| (2) |
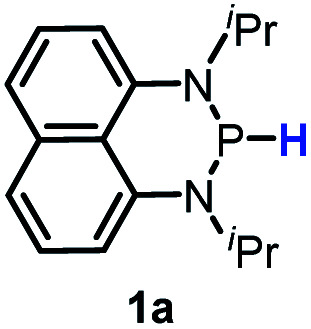
|
tBuOK, CD3CN, 20 °C, 10 min |
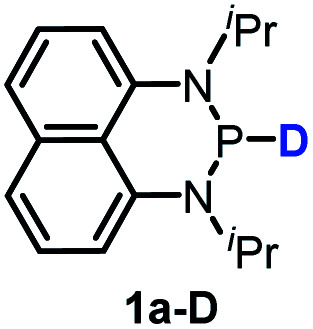
|
Quant.b | PT |
| (3) |
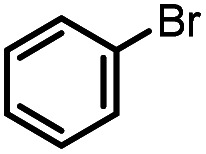
|
AIBN (1.5 equiv.), C6D6, 80 °C, 3 h |
|
>90%b | HAT |
| (4) |
|
1b (1.5 equiv.), AIBN 15 mol%, toluene-d8, 90 °C, 5 h |
|
>90%c | HAT&ET |
Isolated yield.
NMR yields determined by the amount of phosphorus species.
NMR yields with 1,3,5-trimethoxybenzene as the internal standard.
