Figure 5.
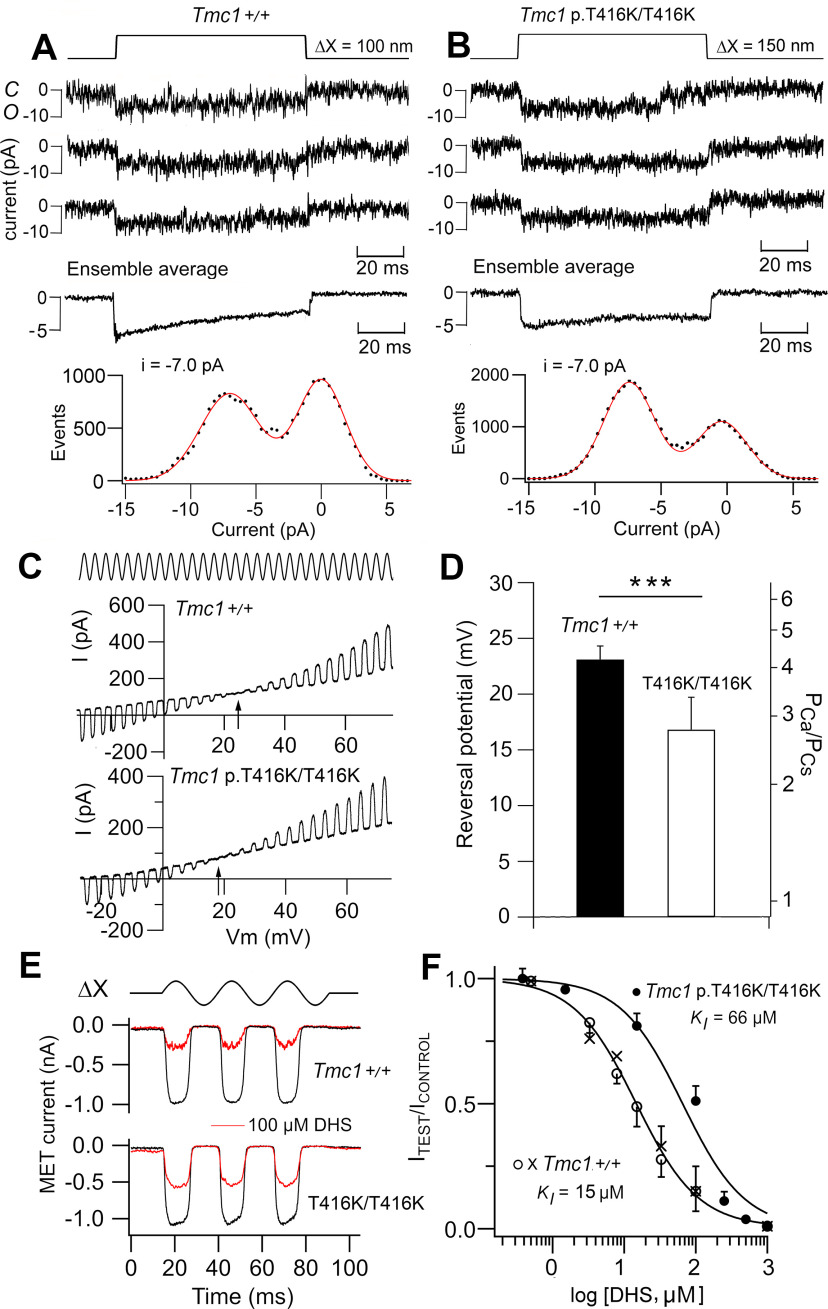
Ionic properties of MET channels from apical OHCs of Tmc1 p.T416K/T416K; Tmc2−/−. A, Three examples of single MET channel currents for Tmc1+/+; Tmc2−/−, ensemble average of 30 presentations (middle) and amplitude histogram (bottom) giving −7.0 pA at –84 mV. C and O indicate closed and open states of channel. B, Examples of single MET channel currents for Tmc1 p.T416K/T416K; Tmc2−/−, ensemble average of 100 presentations (middle) and amplitude histogram (bottom) giving −7.0 pA at −84 mV. C, Determination of MET channel Ca2+ permeability in isotonic external Ca2+ and internal Cs+ (see Materials and Methods). During a sinusoidal bundle vibration (ΔX, ±200 nm), the membrane potential (Vm) was swept from −30 to +80 mV and the MET current reversed polarity at arrowed potential. Reversal potential in Tmc1+/+; Tmc2−/− is ∼7 mV positive to that in Tmc1 p.T416K/T416K; Tmc2−/−. D, Collected reversal potentials and relative permeability PCa/PCs in six cells mean +/− SD. Control and mutant are significantly different (t test, ***p < 0.001). E, MET currents for bundle vibrations (ΔX, ±200 nm) in normal saline (black traces) and in presence of 100 μm DHS in Tmc1+/+; Tmc2−/− (top) and Tmc1 p.T416K/T416K; Tmc2−/− (bottom). F, Mean ± SD (N = 5) of MET current block by DHS in Tmc1+/+ and Tmc1 p.T416K/T416K. Hill plots give KI = 15 μm (all points) and 66.0 μm, respectively, with Hill coefficients = 1 for both genotypes. For control curve, open symbols determined using bundle stimulation with a fluid et (KI = 14.0 μm), whereas crosses obtained with a stiff glass probe (KI = 15.4 μm). The similar KI values for the different jstimulation methods rules out artifacts because of dilution of DHS by saline in the fluid jet.