Optimization studies of the coupling of ketimine 1a and tetrahydrofuran 2aa,b.
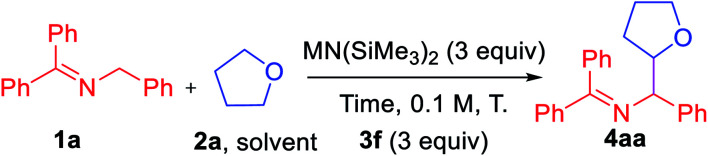
| ||||
|---|---|---|---|---|
| Entry | M = | Time/h | T/°C | Yield of 4aa/% |
| 1 | Li | 24 | 80 | 49 (dr = 3.4 : 1) |
| 2 | Li | 12 | 80 | 58 (dr = 2.8 : 1) |
| 3 | Li | 6 | 80 | 64 (dr = 2.7 : 1) |
| 4 | Li | 3 | 80 | 68 (dr = 2.8 : 1) |
| 5 | Li | 1 | 80 | 73 (dr = 2.8 : 1) |
| 6 | Na | 1 | 80 | 81 (dr = 1.6 : 1) |
| 7 | K | 1 | 80 | Complex mixture |
| 8 | Na | 1 | 100 | 52 (dr = 1.5 : 1) |
| 9 | Na | 1 | 60 | 69 (dr = 1.7 : 1) |
| 10c | Na | 1 | 80 | 81 (dr = 1.7 : 1) |
| 11c,d | Na | 1 | 80 | 84 (80)e (dr = 1.7 : 1) |
Reactions conducted on a 0.1 mmol scale. Assay yields determined by 1H NMR spectroscopy of the crude reaction mixture using C2H2Cl4 as an internal standard.
Diastereomeric ratio (dr) of alpha coupling product between 1a and 2a determined by HPLC. The beta coupling product was observed in trace amounts by HPLC but the dr could not be determined.
0.2 M.
3f (2 equiv.).
Isolated yield and diastereomeric ratio after chromatographic purification.
