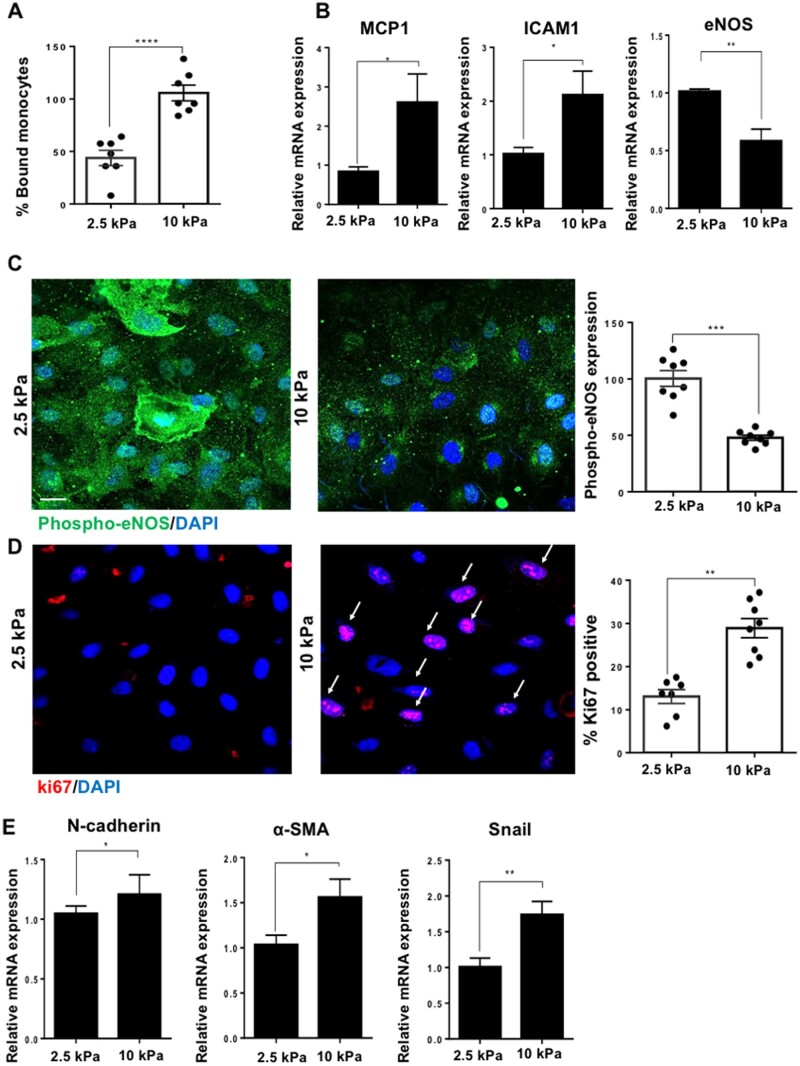
Figure 3.
Stiffness promotes EC dysfunction by increasing inflammation, proliferation, EndMT, and by inhibiting nitric oxide signalling. Cells were cultured on either 2.5 or 10 kPa gels until confluent (48 h). (A) Cell inflammation was assessed by a monocyte adhesion assay and data were quantified as percentage bound monocytes on the surface of ECs, N = 7. (B) Inflammatory marker gene expression was assessed by qPCR. HPRT was used as a housekeeping gene, N = 6. (C) To assess active nitric oxide signalling, we assessed the expression of phosphorylated-eNOS (phospho-eNOS, green) and nuclei were identified using DAPI (blue). The expression of phospho-eNOS was quantified as fluorescent intensity and was normalized as a percentage (expression in 10 kPa normalized to 2.5 kPa) N = 8. (D) Cell proliferation was assessed by ki67 (red) immunostaining and nuclei were stained with DAPI (blue). Arrows indicate ki67-positive nuclei. The data were quantified as percentage positive cells for ki67, N = 7–8. Scale bar= 20 μm. (E) Endothelial–mesenchymal transition (EndMT) gene marker expression was assessed by qPCR, N = 9. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01, and ***P < 0.001 using a Student’s t-test.