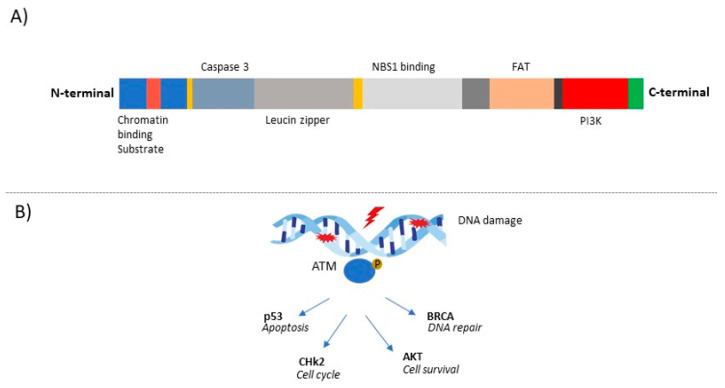
Figure 1.
Structure of human ATM and function in DNA damage repair. (A) ATM is characterized by an N-terminal and a C-terminal half that shows homology to other phosphoinositide-3 kinase (PI3K)-like kinases and a portion that contains a FAT domain (named after the FRAP, ATR, and TRRAP proteins). The N-terminal portion interacts with substrates and cofactors such as NBS1, p53, and BRCA1. In addition, the N-terminal portion is characterized by a proposed chromatin- interaction domain, a nuclear localisation sequence (NLS), two caspase-3 cleavage sites, and a putative leucine zipper region. (B) Following DNA damage, ATM is recruited and is catalytically activated by autophosphorylation. Once activated, ATM serves as a transducer, phosphorylates, and activates other protein kinases such as checkpoint kinase 2 (CHK2), which in turn modulates its own substrates, resulting in cell cycle arrest, or ATM can also activate p53. ATM can activate mechanisms involved in DSB repair, such as BRCA proteins.