Optimization of the propellylation/borylation reaction. [1.1.1]propellane (1, 1.4 equiv.), reaction concentration = 0.2 M.
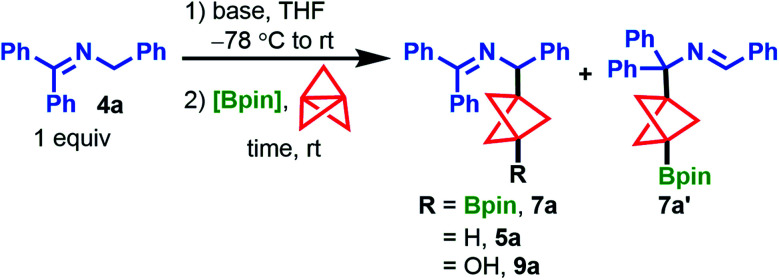
| ||||||
|---|---|---|---|---|---|---|
| Entry | Base (equiv.) | Bpin source (equiv.) | Time (h) | % 7aa | % 4aa | % 5a : 9a : 7a′a |
| 1b | LiN(SiMe3)2 (2) | B2pin2 (2) | 4 | 43 | 0 | 13 : 0 : 5 |
| 2b | LiN(SiMe3)2 (1) | B2pin2 (2) | 7 | 55 | 0 | 18 : 0 : 6 |
| 3b | NaN(SiMe3)2 (2) | B2pin2 (2) | 24 | 25 | 0 | 70 : 0 : 4 |
| 4b,c | n-BuLi (1.1) | B2pin2 (2) | 2 | 49 | 6 | 0 : n/d : 4 |
| 5b | n-BuLi (1.1) | iPrOBpin (1.1) | 2 | 33 | 6 | 10 : 27 : 2 |
| 6b | n-BuLi (1.1) | iPrOBpin (5) | 2 | 62 | 19 | 5 : 1 : 6 |
| 7 | n-BuLi (1.1) | iPrOBpin (5) | 2 | 73 | 8 | 0 : 0 : 6 |
| 8 | LDA (1.1) | iPrOBpin (5) | 16 | 74 [61%] | 14 | 0 : 0 : 7 |
| 9d | LDA (1.1) | iPrOBpin (5) | 2 | 60 | 6 | 0 : 0 : 3 |
Determined by 1H NMR analysis of the crude reaction mixture using CH2Br2 as an internal standard.
Base added at room temperature.
1H NMR spectrum of the crude reaction mixture was too complex to accurately quantify 9a and 4a.
Reaction initiated at −78 °C and warmed to 50 °C.
