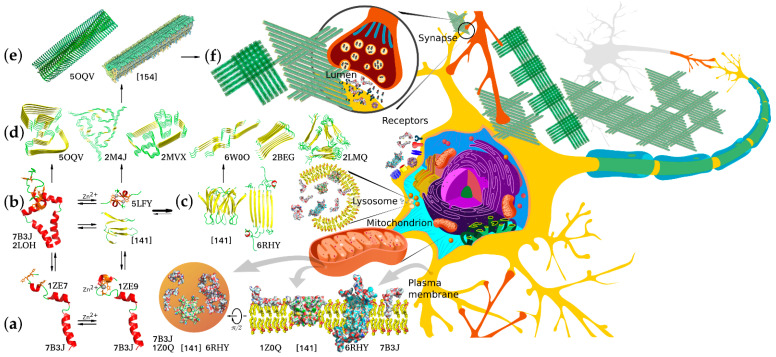
Figure 2.
Structural diversity of Aβ aggregation states, aggregation pathways, and examples of biologically relevant interaction targets. Corresponding PDB accession numbers are indicated alongside the diagrams. (a) Free and cation-bound monomeric Aβ peptides (the structure combined from solution NMR structures of folded Aβ1–16 metal-binding domain and Aβ17–42 fragment) are capable of dimerizing via different dimerization interfaces situated in the TM, JM, and metal-binding site regions using protein–protein and protein–cation interactions involving α-helix, β-strand, and random coil structures. (b) Alternative dimers nucleate aggregation into neurotoxic intermediate oligomers that can interact with different target proteins and lipid membranes of neurons. (c) Two known configurations of predominantly β-structured minor oligomers capable of forming pore-like proteolipid aggregates and prone to further aggregation into protofibrillar structures. (d) Diverse structural motifs that can constitute amyloid fibril core structures capable of further aggregating into filaments and fibrillary deposits. (e) Two possible alternative fibril structures with biaxial and triaxial symmetry depositing to form macroscopic aggregates (f) constituting senile plaques, a known hallmark of AD. All the diverse Aβ aggregation forms appear to interact with multiple alternative targets, thus mediating normal physiological functions or pathological processes. The interactions are known to occur in multiple morphological units and organelles, including plasma and synaptic membranes and inter- and intracellular components.