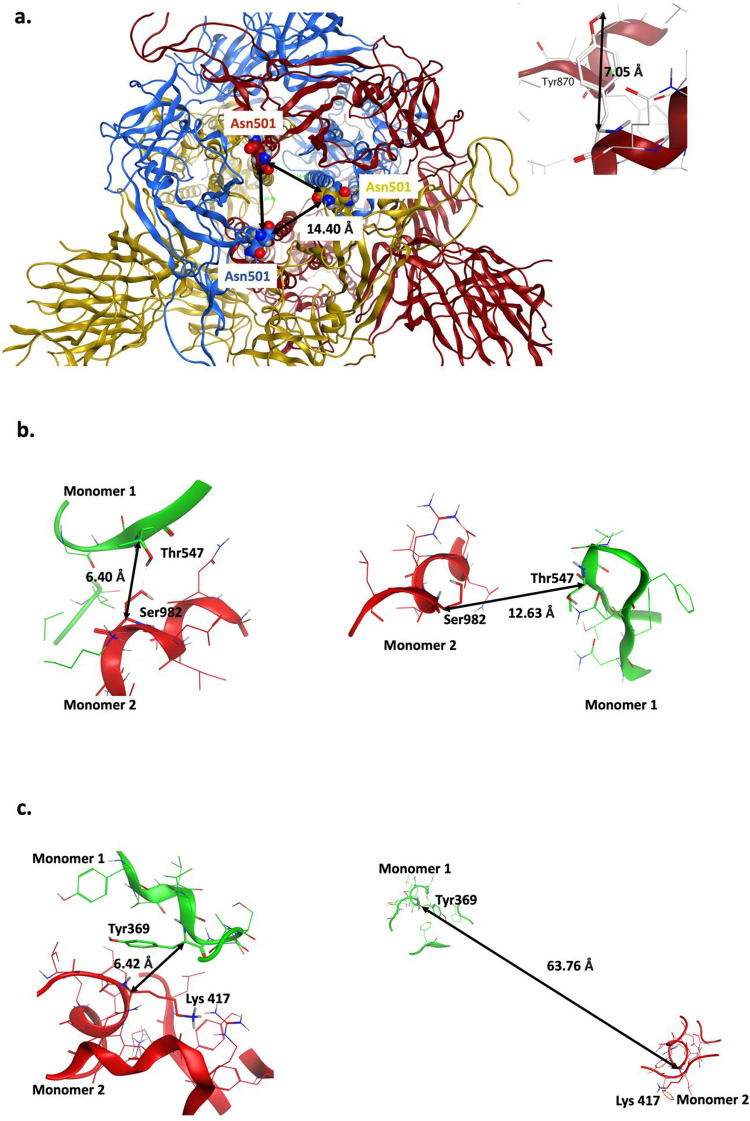
Fig 3. Potential destabilizing effects of the point mutations on the closed conformation of the spike protein trimer.
(a) Distance between the backbones of Asn501 in closed conformation is shown (left) and typical length of Tyr side chain is shown (right). Asn501Tyr mutation might introduce steric clashes and destabilize the closed conformation. (b) Ser982 (monomer 2)-Thr547 (monomer 1) interaction in closed (left) and open (right) conformation. Ser982Ala results in loss of this inter-monomeric interaction. (c) Lys417 (monomer 2)-Tyr369 (monomer 1) interaction in closed (left) and open (right) conformation. Lys417Asn mutation may reduce this inter-monomeric interaction.