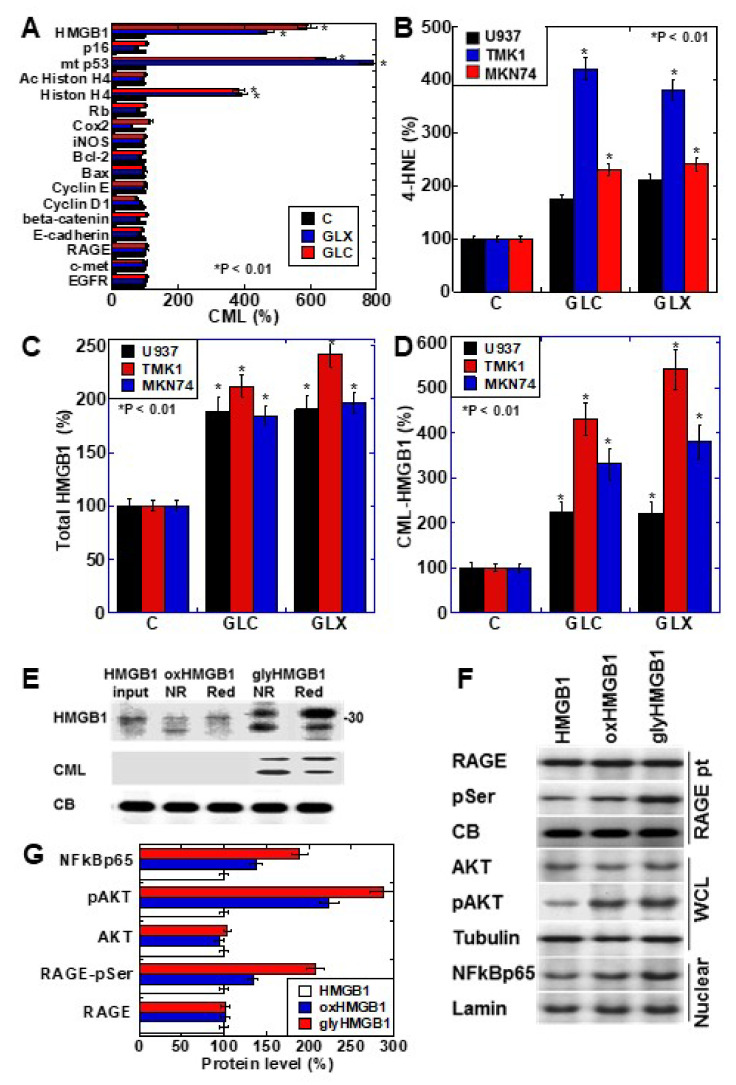
Figure 1.
CML-HMGB1 formation in gastric cancer cells. (A) CML formation in various cancer-associated proteins was analyzed by antibody array using immunoprecipitant by anti-CML antibody of TMK-1 cell lysate, which was treated with GLX or GLC. The Scheme 100. (B) Oxidative stress levels in cells treated with GLX or GLC as measured by ELISA. (C) Total HMGB1 levels in cells treated with GLX or GLC as measured by ELISA. (D) CML-HMGB1 levels in cells treated with GLX or GLC measured by immunoprecipitation; immunoprecipitant by anti-CML antibody was detected by anti-HMGB1 antibody. (E) H2O2-treated HMGB1 (oxidized HMGB1) and GLX-treated HMGB1 (glycated HMGB1) were examined by Western blot analysis under non-reduced (NR) or reduced (Red) conditions. The membrane was re-probed with an anti-CML antibody (CML). (F) Alterations in HMGB1-associated intracellular signals in TMK-1 cells treated with naïve HMGB1 (10 μg/mL), oxidized HMGB1 (10 μg/mL), or glycated HMGB1 (10 μg/mL). (G) Semi-quantification of protein levels with standardization by CB, tubulin, or lamin. Phosphorylated RAGE (pSer) was examined by immunoprecipitation; RAGE precipitant was detected using an anti-pSer antibody. CB, tubulin, and lamin were used as the loading controls. Error bar and standard deviation calculated by ordinary analysis of variance from three independent experiments. CML, Nε-(Carboxymethyl)lysine; HMGB1, high-mobility group box-1; C, untreated control; GLX, glyoxal; GLC, glucose; RBP, retinol-binding protein; 4-HNE, 4-hydroxynonenal; NR, non-reduced condition; Red, reduced condition; CB, Coomassie blue; RAGE, receptor for advanced glycation end products; pSer, phosphorylated serine; pAKT, phosphorylated AKT; .NF-κB, nuclear factor-κB; RAGE pt, anti-RAGE antibody precipitant; WCL, whole cell lysate; Nuclear, nuclear fraction. * P < 0.01.