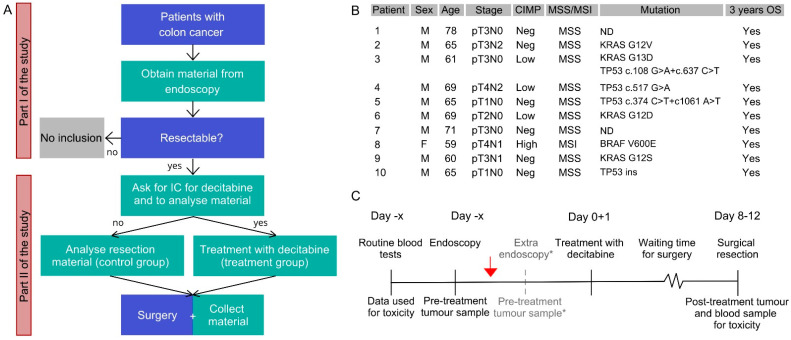
Figure 1.
(A) Flow chart of DECO study. In blue squares, daily routine steps in clinical care are shown; in green squares, additional steps for DECO study are shown; (B) Baseline characteristics of decitabine-treated patients included in DECO study. Indicated stage is pathological staging after resection was done. Initial staging (for inclusion) was performed based on CT scan; (C) Timeline for DECO study. Red arrow shows the moment of inclusion. Blood tests before inclusion were part of standard of care. * For the patients with fresh frozen material, an extra endoscopy was performed to obtain freshly frozen biopsies as pre-treatment sample. For FFPE patients, pre-treatment samples were obtained from the diagnostic endoscopy performed for clinical purposes. IC = informed consent, ND = not detected, OS = overall survival.