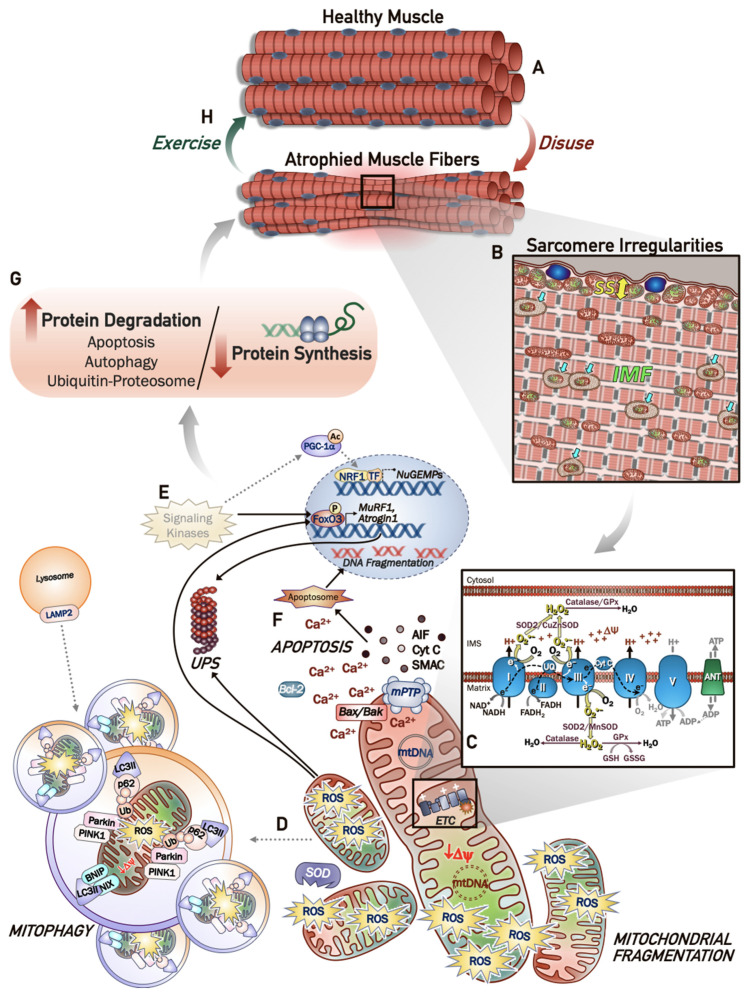
Figure 1.
Disuse-induced mitochondrial derangements contribute to skeletal muscle fiber atrophy. (A) Atrophic regions of muscle fibers brought about following periods of prolonged muscle inactivity display marked alterations in mitochondrial morphology and function which can be visualized microscopically and have been determined experimentally. (B) Both subsarcolemmal (SS; yellow arrow) and intermyofibrillar (IMF) mitochondrial populations appear fragmented, with irregular shapes and unusual cristae formation indicative of dysfunctional organelles (green mitochondria). Defects in the mitochondrial turnover machinery contribute to the appearance of undegraded vacuoles, termed lipofuscin (blue arrows), which contribute to the irregularities within the sarcomere. (C) The absence of muscle contraction results in a decrease in the ADP supply, resulting in lower respiratory rates and an enhanced proton motive force (Δψ), thus promoting the creation of reactive oxygen species (ROS) in the form of superoxides (O2•−) and H2O2. Mitochondrial and cytosolic antioxidants neutralize superoxides, first via conversion to H2O2 by superoxide dismutases SOD1 and SOD2, then to water by antioxidant enzymes such as catalase or glutathione. As mitochondrial antioxidants are downregulated during muscle disuse, ROS accumulate in muscle mitochondria triggering the activation of degradation pathways. (D) Mitochondrial quality is maintained by the mitophagy machinery to selectively envelop dysfunctional organelles and delivers them to the lysosome for recycling. With chronic disuse, the process is impaired, leading to the accumulation of undegraded dysfunctional mitochondria. (E) In addition to this impaired turnover, activation of signaling kinases is reduced, leading to a diminished drive for mitochondrial biogenesis via PGC-1α, and promoting the expression of atrogenes, such as MuRF1 and atrogin1 that enhance protein degradation. (F) A consequence of the impaired mitochondrial quality control and chronic organelle dysfunction is the formation and opening of the mitochondrial permeability transition pore (mPTP), and subsequent release of pro-apoptotic factors such as AIF, Cytochrome c and SMAC, which lead to DNA fragmentation. (G) Collectively, these derangements contribute to enhanced protein degradation relative to protein synthesis, thus, exacerbating muscle atrophy. (H) Regular endurance exercise reverses many of the mitochondrial defects that are observed with chronic inactivity, and therefore is capable of preventing, diminishing or reversing disuse-induced muscle atrophy.