Fig. 7. Model of mitochondrial ISR regulation by CHOP-CEBPβ-ATF4 interplay.
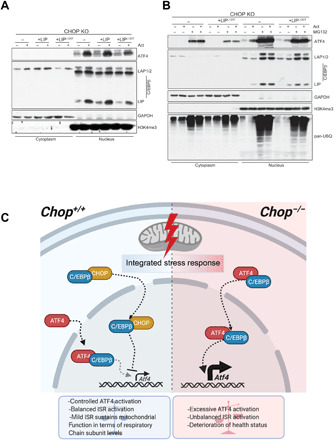
(A) Cell fractionation followed by the Western blot analysis of the ATF4 and three C/EBPβ isoforms in actinonin-treated (48 hours) WT or CHOP KO MEFs expressing WT C/EBPβ LIP or C/EBPβ LIPL120T mutant. (B) Cell fractionation followed by the Western blot analysis of the ATF4 and three C/EBPβ isoforms in actinonin-treated (48 hours) CHOP KO MEFs expressing WT C/EBPβ LIP and C/EBPβ LIPL120T mutant along with the WT cells and respective controls. The MG132 (15 μM) was applied in the last 6 hours of the actinonin treatment. Elevated protein ubiquitination reflects proteasome inhibition. (A and B) Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and H3K4me3 were used as loading controls and to determine quality of fractionation (n = 3). (C) CHOP levels increase early upon mitochondrial dysfunction leading to its association with C/EBPβ. The interaction with C/EBPβ likely promotes translocation of CHOP to the nucleus where it negatively regulates Atf4 levels and transcription of downstream ISR targets. Abrogation of CHOP results in increased ATF4:C/EBPβ association and transcription of ISR-regulated genes, created with BioRender.com.
